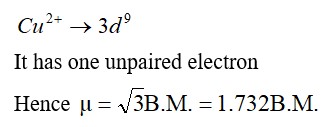Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Potassium permanganate in alkaline medium oxidise lodide to lodate.
Compound A is
New answer posted
4 months agoContributor-Level 10
KMnO4 decomposes upon heating at 513 K and forms K2MnO4 and MnO2.
2KMnO4
New answer posted
4 months agoContributor-Level 10
The colour changes due to change in pH not due to its reaction with water.
New answer posted
4 months agoContributor-Level 10
Each of the element in group III
Mn is in group seven shows a maximum oxidation state of +7
New answer posted
4 months agoContributor-Level 10
Zn2+ has no unpaired electron and colourless in aqueous solution.
New answer posted
4 months agoContributor-Level 10
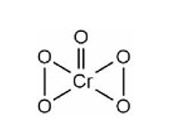
This is an example of chromyl chloride test
K2Cr2O7 + 4KCl + 6H2SO4? 6KHSO4 + 2CrO2Cl2 + 3H2O
Oxidation state of Cr is +6.
New answer posted
4 months agoContributor-Level 10
A, B and C are correct.
Mn2O7 is a green oil at room temperature.
V2O4 react with acids to give VO2+.
CrO is Basic and CrO3 is acidic.
V2O5 react with acids as well as alkali.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers