Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
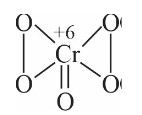
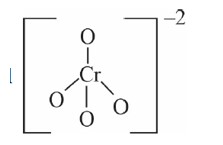
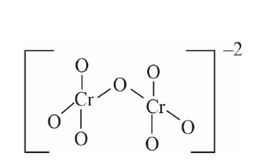
Chromate ion shape tetrahedral
But dichromate ion has only one common oxygen bond.
New answer posted
5 months agoContributor-Level 10
A, B and C can be precipitated by HCl as well as H2S as their insoluble chlorides and sulphides.
(D) Sn+2 can be precipitated by H2S but not by HCl
New answer posted
5 months agoContributor-Level 10
When Ferric chloride reacts with potassium thiocyanate a blood red colour of Ferric thiocyanate is formed
FeCl? + 3KSCN → Fe (SCN)? + 3KCl
New answer posted
5 months agoContributor-Level 10
To stop bleeding, FeCl? is applied locally because FeCl? causes denaturation of proteins present in blood. It is a case of coagulation.
New answer posted
5 months agoContributor-Level 9
the strongest oxidizing agent have the highest reduction potential. So Mn3+ is the strongest oxidizing agent.
New answer posted
5 months agoContributor-Level 9
Cerium exists in two oxidation states (+3) and (+4)
It exist as Ce+4 and acts like a strong oxidizing agent by gaining electrons
New answer posted
5 months agoContributor-Level 10
Cu is the only element of 3d – series whose M2+ / M value is positive because of fact that low hydration enthalpy and high sublimation & ionization enthalpies.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers