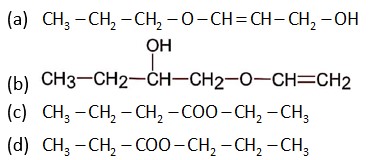Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Here A is CH3 - CH2 – CH2 – COO – CH2 – CH3
The given sequence of reaction is:
New answer posted
6 months agoContributor-Level 10
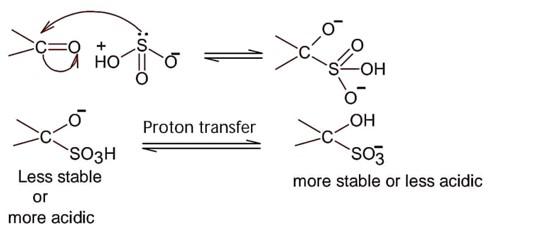
Nucleophilic addition of sodium hydrogen sulphite to aldehyde or ketone is a;
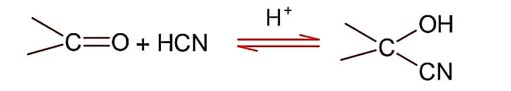
So; nucleopilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Addition of hydrogen cyanide;
Final product is cyanohydrin.
New answer posted
6 months agoContributor-Level 9
La+2 (z = 57) = 5d1
Ce+2 (z = 58) = 4f2
Nd+2 (z = 60) = 4f4
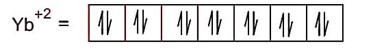
Yb+2 (z = 70) = 4f14
Yb+2 has no unpaired electrons thus diamagnetic in nature.
New answer posted
6 months agoContributor-Level 10
Mn6+ has one unpaired electron so paramagnetic and has green colour.
New answer posted
6 months agoContributor-Level 10
MnO -> Antiferromagnetism
O2 -> Paramagnetism
NaCl -> Diamagnetism
Fe3O4 -> Ferrimagnetism
New answer posted
6 months agoContributor-Level 10
V2O3 – Basic
CrO – Basic
Metal oxides are generally basic in nature but if more electronegative element are connected through central atom then deficiency of electron occurs on central atom and it behaves as acidic in nature.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers