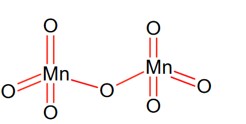
Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
According to the John-Tollen distortion, there is a unsymmetrical filling of eq set of Cu+= ions but there is symmetrical filling of t2g set of ions.
New answer posted
6 months agoContributor-Level 10
Following are the
Cr2+ - 1926 KJ/mole
Mn2+ - 1860 KJ/mole
Fe2+ - 2000 KJ/mole
Co2+ - 2078 KJ/mole
New answer posted
6 months agoNew answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
Following are the
Cr2+ - 1926 KJ/mole
Mn2+ - 1860 KJ/mole
Fe2+ - 2000 KJ/mole
Co2+ - 2078 KJ/mole
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers