Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
8.34 From the table given below:
Mn3+ : 3d4 | unpaired electrons = 4 |
V3+ : 3d2 | unpaired electrons =2 |
Cr3+ : 3d3 | unpaired electrons= 3 |
Ti3+ : 3d1 | unpaired electrons =1 |
Cr3+ is most stable in aqueous solution because of half filled d-orbital.
New answer posted
9 months agoContributor-Level 10
8.33 Copper (29) has electronic configuration 1s22s22p63s23p63d104s1. It can easily lose one electron to give stable configuration as it has completely filled d-orbital.
New answer posted
9 months agoContributor-Level 10
8.32 The reactions in which same substance gets oxidized as well as reduced due to unstable oxidation state.
Example:
2MnO42- + 4H+? 2MnO4- + MnO2 + 2H2O
Mn (VI) is oxidised to Mn (VII) and also reduced to Mn (IV).
2CrO4 3- +2H+? CrO4 2- +Cr3+ + 4H2O
Cr (V) is oxidised to Cr (VI) and also reduced to Cr (III).
New answer posted
9 months agoContributor-Level 10
8.31 (i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidizing: The +2 oxidation state becomes more stable on moving across a period i.e. the tendency of metals to give electrons becomes more. Therefore, vanadium(II) oxide and chromium(II) oxide are strong reducing agents.
As if the value of electrode potential is higher i.e. more energy required to withdraw an electron from an isolated atom, more readily it can be reduced and lesser the electrode potential more readily it can be oxidized.
The electrode potential of Cr3+? Cr2+is negative so it acts reducing agent or can undergo oxidation which makes it
New answer posted
9 months agoContributor-Level 10
8.30
| Lanthanoids | Actinoids |
Electronic Configuration | It is represented by [Xe]4fx5dy6s2, where x varies from 0 to 14 and y= 0 or 1 | It is represented by [Rn]5fx6dy7s2, where x varies from 0 to 14 and y= 0 or 1. |
Oxidation state | Generally shows +3 oxidation state only in some cases it is +2 or +4 but never greater than +4 | It has +3 oxidation state but also shows higher oxidation states such as +4, +5, +6, +7. |
Atomic and ionic sizes | Ionic radii of M3+ions decrease in size with increase in atomic number this is called as lanthanoid contraction. | There is a gradual decrease in the size of M3+ ions across the series, this is known as actinoid contraction. |
Chemical Reactivity | Lanthanoids are less reactive in nature and form oxides, sulphides, nitrides etc. They have a lesser tendency to form complexes. | They are highly reactive in nature when they are in the finely divided state. They have a higher tendency to form complexes and even react with non-metals at moderate temperature. |
Actinoid Contraction > Lanthanoid contraction
Lanthanoids show lanthanoid contraction due to which their size is quite small as compared to actinoids although there is actinoid contraction also lanthanoid contraction has more impact on elements as there is one shell less than actinoids, so lanthanoids have less tendency to lose an electron and to undergo any reaction like the formation of oxide etc.
New answer posted
9 months agoContributor-Level 10
8.29 It can be observed from the above table that in the starting of 3d transition series elements like Sc, Ti, V, Cr in +2 state are not that stable in their elements in the +3 state.
In the middle Mn2+, Fe2+, Co2+are quite known. In fact, Mn2+ and Mn7+ are most stable states in Mn. Fe2+ is less stable when compared to Fe3+ which is due to fact that Fe3+ is able to loose one electron to acquire d5 state which has extra stability. Co2+ is less stable as compared to Co3+.
Ni2+ is the most common and stable among its +2, +3, +4 states. Cu2+ is more stable and is quite common as compared to Cu+. Towards the end, Zn forms only Zn2+ whic
New answer posted
9 months agoContributor-Level 10
8.28 Metal ions which have valence electrons in d-orbital and in which d-d transition can take place will be coloured and the metal ions which have completely filled orbital or have d- orbital will be colourless as no d-d transition is possible in those configurations.
Element | Atomic Number | Ionic State | Electronic configuration in ionic state |
Ti | 22 | Ti3+ | [Ar] 3d1 |
V | 23 | V3+ | [Ar] 3d2 |
Cu | 29 | Cu+ | [Ar] 3d10 |
Sc | 21 | Sc3+ | [Ar] |
Mn | 25 | Mn2+ | [Ar] 3d5 |
Fe | 26 | Fe3+ | [Ar] 3d5 |
Co | 27 | Co2+ | [Ar] 3d7 |
From the above table, it can be observed that only Sc3+ and Cu+ have either completely filled d- orbital or empty d-orbital. So, all other ions except Sc3+ and Cu+ will be coloured in aqueous solution because of absorption of radiations which fall in the visible region as on obtaining energy electrons jump from one d-orbital to another.
New answer posted
9 months agoContributor-Level 10
8.27 (i) Reduction potential tells us the ease with which the Metal can get reduced, As E° for Cr3+/Cr2+ is negative (–0.4 V), this means that Cr3+ ions in solution cannot be reduced to Cr2+ ions i.e., Cr3+ ions are very stable. As a further comparison of E° values show that Mn3+ ions more readily than Fe3+ ions which means that Mn3+ is least stable.
So Stability of metal ions is as follows:
Mn3+
(ii) Reduction potential tells us the ease with which the Metal can get reduced or the difficulty with which they are oxidized, As the reduction potential increases in the following order Mn2+/Mn < Cr2+/Cr< Fe2+/Fe
So oxidation of Fe is not as easy
New answer posted
9 months agoContributor-Level 10
8.26 Potassium permanganate can be prepared from MnO2. It can be done by fusing the ore with KOH in presence of an oxidizing agent like atmospheric oxygen/KNO3 etc to give green coloured K2MnO4 as the product.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
The Product K2MnO4 is extracted with water and then oxidised by passing ozone/chlorine into the solution or electrolytically.
Electrolytic oxidation:
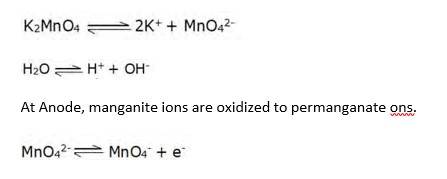
(i) Acidified KMnO4 solution oxidizes Fe(II) ions to Fe(III) ions and water as product
MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O
Fe2+→ Fe3++ e- } x5
Overall: MnO4 - + 8H+ + 5Fe2+ → Mn2+ + 4H2O + 5Fe3+
(ii) Acidified potassium permanganate oxidizes SO2 to su
New answer posted
9 months agoContributor-Level 10
8.25 K2Cr2O7 acts as a very strong oxidizing agent in acidic medium. K2Cr2O7 gets reduced and acts as an oxidizing agent by oxidizing Iodide to iodine
(i) K2Cr2O7 oxidizes iodide to iodine
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
2I-→ I2 + 2e- } x3
Overall: Cr2O72-+ 14H++ 6I-→ 2Cr3+ + 7H2O+ 3I2
(ii) K2Cr2O7 oxidizes iron(II) to iron(III)
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
Fe2+→ Fe3+ + e- } x6
Overall: Cr2O72-+ 14H++ 6Fe2+→ 2Cr3+ + 7H2O + 6Fe3+
(iii) K2Cr2O7 oxidized H2S to sulphur
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
H2S → S + 2H+ + 2e-} x3
Overall: Cr2O72-+ 8H++ 3H2S → 2Cr3+ + 7H2O +3S
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
