Block D and F Elements
Get insights from 162 questions on Block D and F Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about Block D and F Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
8.24 Potassium dichromate is prepared from chromite ore (FeCr2O4) by the following steps:
Step1: Preparation of sodium dichromate in the reaction of Chromite ore with sodium hydroxide and oxygen gas.
4FeCr2O4 + 16NaOH + 7O2? 8Na2CrO4 + 2Fe2O3 + 8H2O
Step2: Conversion of Sodium Chromate on reaction with concentrated Sulfuric acid gives Sodium dichromate as a product.
2Na2CrO4 + conc. H2SO4? Na2Cr2O7 + Na2SO4 + H2O
Step3: Sodium dichromate on reaction with potassium chloride converts to potassium dichromate as a product.
Na2Cr2O7 + 2KCl? K2Cr2O7 + 2NaCl
As potassium dichromate is less soluble than sodium chloride so, potassium dichromate
New answer posted
9 months agoContributor-Level 10
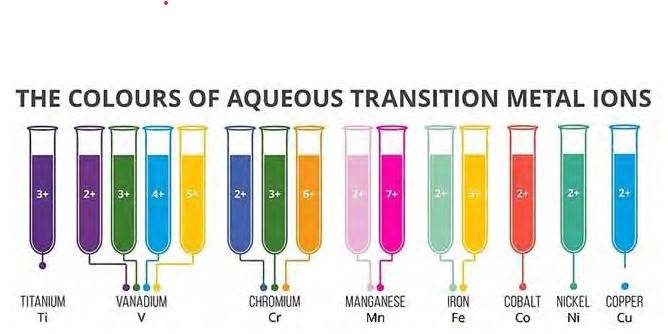
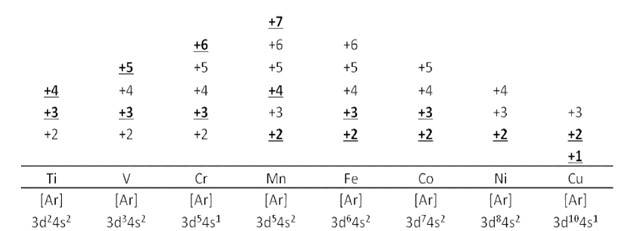
8.23 In transition metals, it can be observed that the oxidation state of the metal varies from +1 to +7 which is obtained by removing all its valence electrons. In transition elements the oxidation state differ by 1 unlike non transition elements whose oxidation state differs by 2, for example in transition metals (Cu2+, Cu3+ and Fe2+, Fe3+) and In non-transition elements, this variation is selective, always differing by 2, e.g. S exhibits +2, +4, +6 oxidation states and N exhibits +3, + 5, etc.
New answer posted
9 months agoContributor-Level 10
8.22 Interstitial compounds are those compounds in which small atomic size elements like H, C, N occupy the interstitial sites of the crystal lattice of the Transition metal due to their large size.
Example, in the diagram given below:
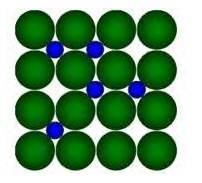
Here, Green Balls form the Crystal lattice of transition metal and Blue balls represent elements like H/C/N which occupy the interstitial sites.
New answer posted
9 months agoContributor-Level 10
8.21 (i) Transition metals and many of their compounds show paramagnetic behavior- Paramagnetic behaviour is shown by transition metals as paramagnetism is due to the presence of unpaired electrons which have a magnetic moment associated with its spin and angular momentum, as the orbital angular momentum is satisfied in the first transition series. So the paramagnetic is only due to the unpaired electrons.
(ii) The enthalpies of atomisation of the transition metals are high- Enthalpies of atomization is the enthalpy change when 1 mol of gaseous atoms is formed from its element in its defined physical state under standard cond
New answer posted
9 months agoContributor-Level 10
8.20 +3 oxidation state is the most common oxidation state of lanthanides i.e., Ln (III) compounds are predominant. +2 and +4 oxidation states can also be found in the solution or in solid compounds, but are not predominant.
New answer posted
9 months agoContributor-Level 10
8.19 Transition metals have a partially filled d−orbital. Therefore, the electronic configuration of transition elements is (n−1) d1-10 ns0-2 The non-transition elements either do not have a partially filled d−orbital. Therefore, the electronic configuration of non-transition elements is ns1-2 orns2 np1-6.
New answer posted
9 months agoContributor-Level 10
8.18 Transition elements are those elements in which the atoms or ions (in stable oxidation state) contain partially filled d-orbital. These elements in the d-block show a transition of properties between s-block and p-block. Therefore, these are called transition elements. Elements such as Zn, Cd, and Hg cannot be classified as transition elements because these have completely filled d-subshell.
New answer posted
9 months agoContributor-Level 10
8.17 On moving along the lanthanoid series, the atomic number increases. Also, with the increase in atomic number, the number of electrons in the 4f orbital also increases.
The 4f electrons have a poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. Consequently, the force of attraction between the nucleus and valence electrons increases. This results in a decrease in the size of lanthanoids with the increase in the atomic number. This is termed as lanthanoid contraction.
CONSEQUENCES:
1. The properties of second and third transition series are similar in
2. Separation of lanthan
New answer posted
9 months agoContributor-Level 10
8.16 (i) Vanadate (VO3 -)-Oxidation state of V is +
(ii) Chromate (CrO4 2-)-Oxidation state of Cr is +
(iii) Permanganate (MnO4 -) Oxidation state of Mn is + 7
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers