Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Get insights from 97 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
A - B has the lowest potential energy and it means it has stronger bond.
New answer posted
6 months agoContributor-Level 10
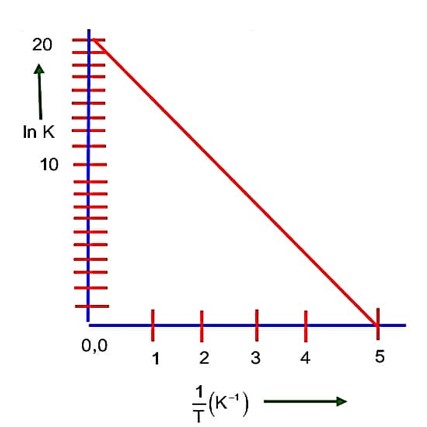
Rate constant, k = 5.5 * 10-14 s-1.
=
From (i) & (ii)
= 1.58 t50%
So; t67% is 15.8 * 10-1 times half life.
X = 16 (the nearest integer)
New answer posted
6 months agoContributor-Level 10
Here, meq of MnO2 = meq of Na2S4O6
Mass of MnO2 in sample = 0.261 g
Percentage of MnO2 in sample =
= 13.05%
New answer posted
6 months agoContributor-Level 10
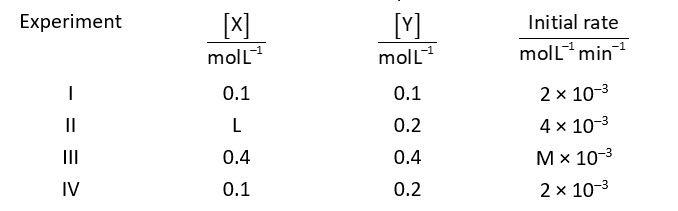
According to condition
Rate
By using experimental data I and II
And by using experimental data I and II
M = 8
Ratio of
New answer posted
6 months agoContributor-Level 10
SCl2 – 2 lone pair of central atom
O3 – 1 lone pair of central atom
ClF3 – 2 lone pair of central atom
SF6 – 0 lone pair of central atom
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers