Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Get insights from 97 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: B, C and D
Catalysts reduce the activation energy of a process and give an alternate path by reducing or raising the activation energy between reactants and products, altering the reaction's enthalpy change. As a result, B, C, and D statements are the proper responses
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and B
Arrhenius equation k = Ae –
When the temperature rises, value falls and –
As a result, e- increases, and it is certain that k and T increases as well. As a result, Option A is proven.
Catalyst increases the rate of reaction by lowering the activation energy. Thus option B is also correct.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
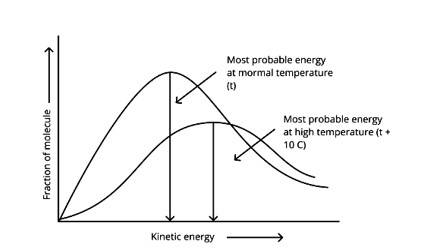
According to the graph, the area under the cure must not vary as the temperature rises.T2 > T1
The energy level of T2 is higher than T1.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and C
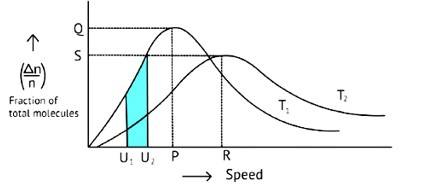
The Maxwell Boltzmann distribution, often known as the Gibbs distribution, is a measure that expresses the likelihood of a system being in each state as a function of the energy of that state.
Look at the graph.
T2 > T1
The fraction of molecules falls as the temperature rises because the area under the curve shrinks.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
Activation Energy is the amount of energy released when reactant molecules collide and form an activated complex. When energy is released, the complex decomposes into a product.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A, C and D
The pressure in this reaction is extremely high, and it becomes independent of the ammonia concentration. The metal surface becomes saturated with gas molecules when the rate of reaction=Rate constant. The rate of a zero-order reaction is independent of the concentration of reactants in the reaction.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
A complex reaction is one that does not happen in a single step.
The total number of molecules involved in the slowest step of the reaction determines the overall reaction rate.
New answer posted
6 months agoContributor-Level 10
Correct options: A and B
This is a Multiple Choice Questions as classified in NCERT Exemplar
Unimolecular reaction: Unimolecular means relating to a single molecular reaction that describes decomposition of a single reactant.
If product A→ (B)
rate = k [A]
Example Na2O4 (g)→2No2 (g)
Order = 1
Molecularity = 1
The slowest step is the rate determining which determines the Order and Molecularity.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
Elementary reaction: It is a reaction that occurs in a single step.
Here we must understand about the Order and Molecularity.
Molecularity of a reaction = number of reacting species (collide to bring a chemical reaction).
Discussing about the Order, Rate law is used in Order and how do we equate shall be seen in the next step
rate = k [A] α [B] β
For a balanced chemical equation of an elementary reaction Order and Molecularity is same and can never be 0.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options : A, C and D.
Rate law = Experimental rate of the reaction
Suppose aA + bB→cC + dD
Then rate = k [A] α [B] β
Here instead of using complex reaction we use elementary reaction which gets solved in one step
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers