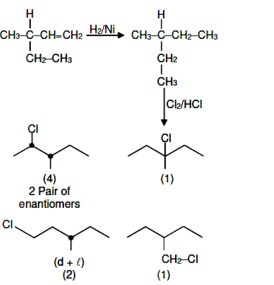
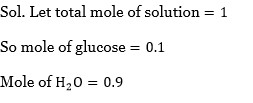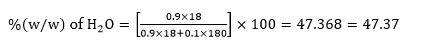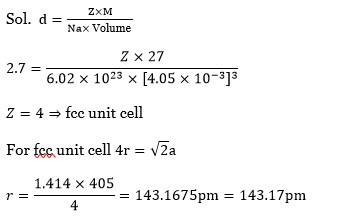Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Get insights from 97 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoBeginner-Level 5
As you know, electrovalent bonds result very strong electrostatic attraction force. All the factors that help maximize this electrostatic attraction are important for the formation of the ionic bond. Here are the important factors;
- Low ionization energy Metal
- High electron affinity Non Metal
- Large-sized cations
- Small-sized anions
- Electronegativity equal to or greater than 1.7
New answer posted
5 months agoContributor-Level 10
Radius of Bohr's orbit
Radius of Bohr's orbit for hydrogen,
For third orbit (R3) = = r3 and
Fourth orbit (R4) =
New answer posted
5 months agoContributor-Level 10
Covalent character is As the cationic size increases polarization decreases.
Covalent character decreases.
New answer posted
5 months agoContributor-Level 10
Covalent character is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers