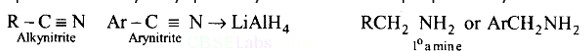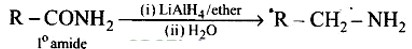Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Get insights from 127 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Aliphatic and arylalkyl primary amines can be prepared by the reduction of the corresponding nitriles with LiAlH4
Heating alkyl halide with primary, secondary and tertiary amine can be prepared by reduction of LiAlH4 followed by treatment with water.
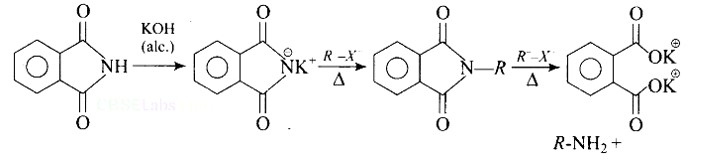
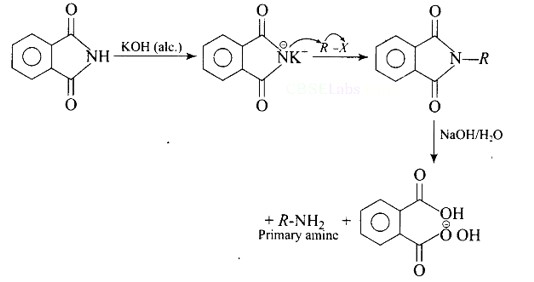
Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis produces primary amine. This process is known as Gabriel phthalimide reaction. The number of carbon atoms in the chain of amines of product is same as reactant.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
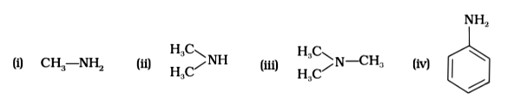
Among primary, secondary, and tertiary amines, tertiary amines are the most volatile compounds as they don't have any strong intermolecular H-bonding between N-H like the primary and secondary amines have.
So due to weak dipole-dipole interactions, (B) will be the most volatile.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A)
The basic strength of an atom is determined by its electron-donating capacity; in this case, the amide is the most basic due to the presence of a negative charge and two lone pairs of electrons on the nitrogen atom.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
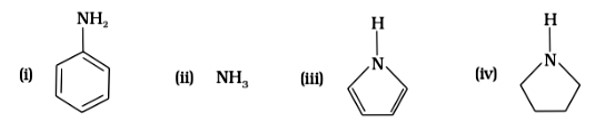
Pyrrolidine is the strongest of two bases because the lone pair of nitrogen does not involve sin resonance, and the presence of two alkyl basic compounds increases the basic strength among the given four compounds.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
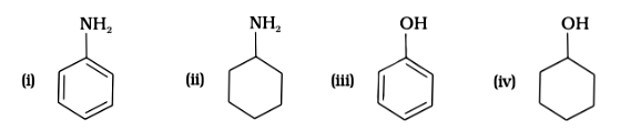
Since phenol is the strongest acid among the four options listed above, it is the weakest Brönsted base. The stronger the acid, the weaker the conjugate base.
Amines have a strong tendency to accept electrons thus they are a strong bronsted base while phenol is the strongest acid among all, therefore as per the relation of conjugative strong acids and weak bases, phenol is the weakest base.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Weak electrophile such as Diazonium cation readily reacts with electron-rich compounds which are having electron-rich compounds such as hydroxyl group, amino group. They don't react with electron-withdrawing groups like nitro groups. Therefore, nitrobenzene will not undergo an azo coupling reaction with benzene diazonium chloride.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
The reaction of transformation of primary alkyl halides to primary amines using potassium phthalimide is the Gabriel phthalimide reaction.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
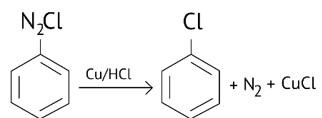
Diazonium salts react with copper powder and halogen acid to form aryl halide.
Gattermann reaction is a Sandmeyer reaction variant in which Cu powder replaces CuCl.
This substitution produces aryl halide more easily and under milder conditions than the Sandmeyer reaction.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A)
On Reaction of acid anhydrides with primary amines, amides are formed as the primary product along with carboxylic acid.
(RCO)2O + RNH2? RCONH2 + RCOOH
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Greater will be the basic character, greater will be its reactivity towards dilute hydrochloric acid. As, (B), secondary amine, it will be the most basic among all and hence the most reactive amine towards acid.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers