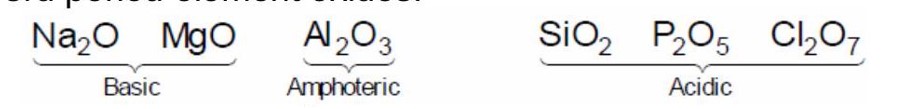Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
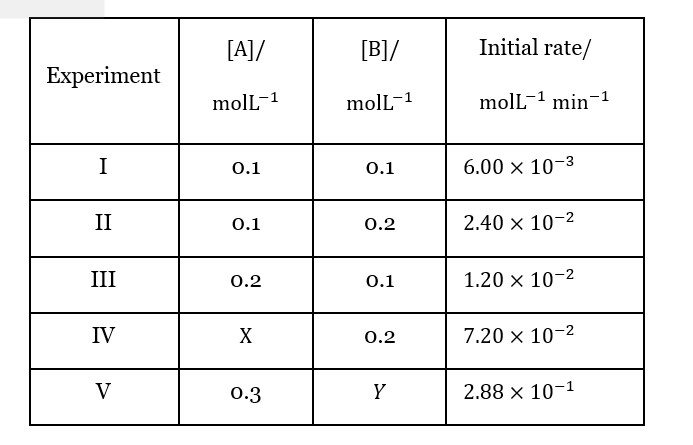
From I&II, rate∝ [B]². From I&III, rate∝ [A]¹.
From IV: 7.2e-2 = k (X) (0.2)². From II: 2.4e-2=k (0.1) (0.2)². X=0.3.
From V: 2.88e-1=k (0.3) (Y)². k=2.4e-2/ (0.1*0.04)=6.
2.88e-1 = 6 (0.3)Y². Y²=0.16. Y=0.4.
New answer posted
4 months agoContributor-Level 10
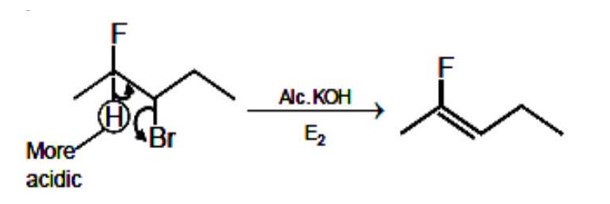
E2 elimination. Most acidic proton is removed. Fluorine is more electronegative.
New answer posted
4 months agoContributor-Level 10
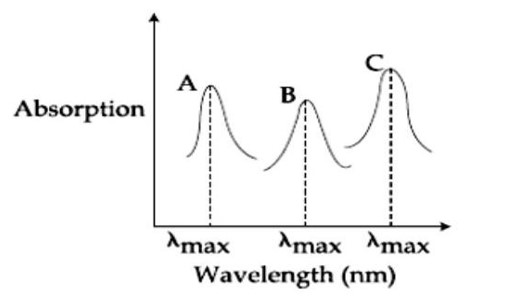
Ligand field strength: NH? > NCS? > F? Stronger ligand, higher Δ, lower λ_max.
So λ (NH? ) < (NCS? ) < (F? ). A= (F? ), B= (NCS? ), C= (NH? ). A-ii, B-i, C-iii.
New answer posted
4 months agoContributor-Level 10
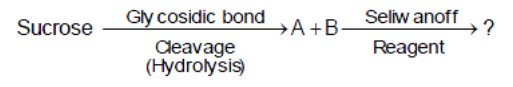
Seliwanoff's test distinguishes aldoses from ketoses. Sucrose hydrolyzes to glucose (aldose) and fructose (ketose). Fructose gives a red color.
New answer posted
4 months agoContributor-Level 10
On moving left to right in a period.
Acidic character of oxides is increase.
3rd period element oxides.
New answer posted
4 months agoContributor-Level 10
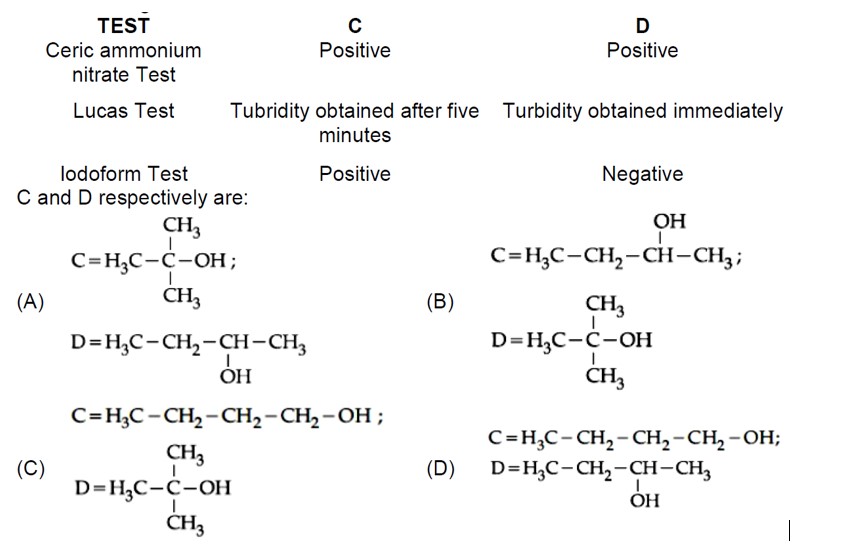
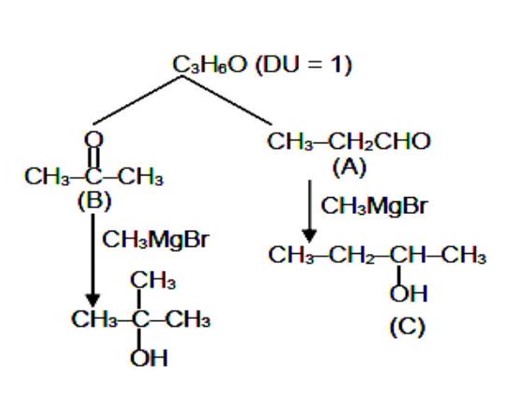
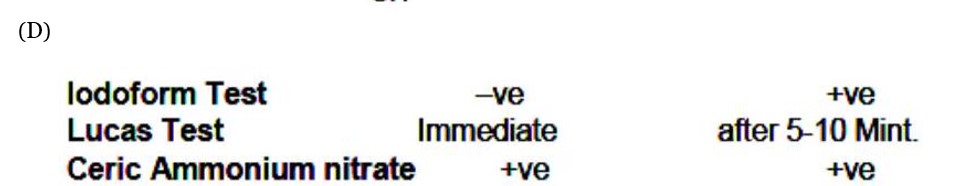
gives iodoform test and slow Lucas test, so it's a methyl secondary alcohol. D gives fast Lucas test, so it's a tertiary alcohol. The Grignard products must be tertiary and secondary alcohols. So A and B must be an aldehyde and a ketone.
New answer posted
4 months agoContributor-Level 10
Li and Mg form nitrides and have bicarbonates that are unstable
New answer posted
4 months agoContributor-Level 10
. [XeF? ]? has 7 electron pairs (5 bonding, 2 lone), pentagonal planar. XeO? F? has 5 electron pairs (trigonal bipyramidal).
New answer posted
4 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers