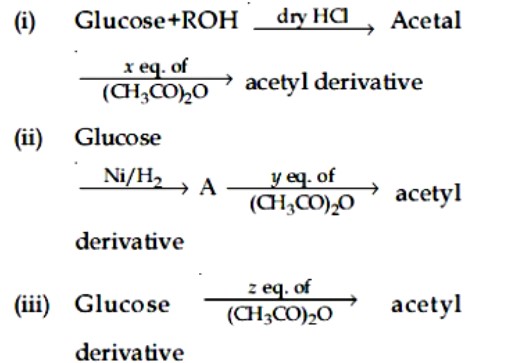
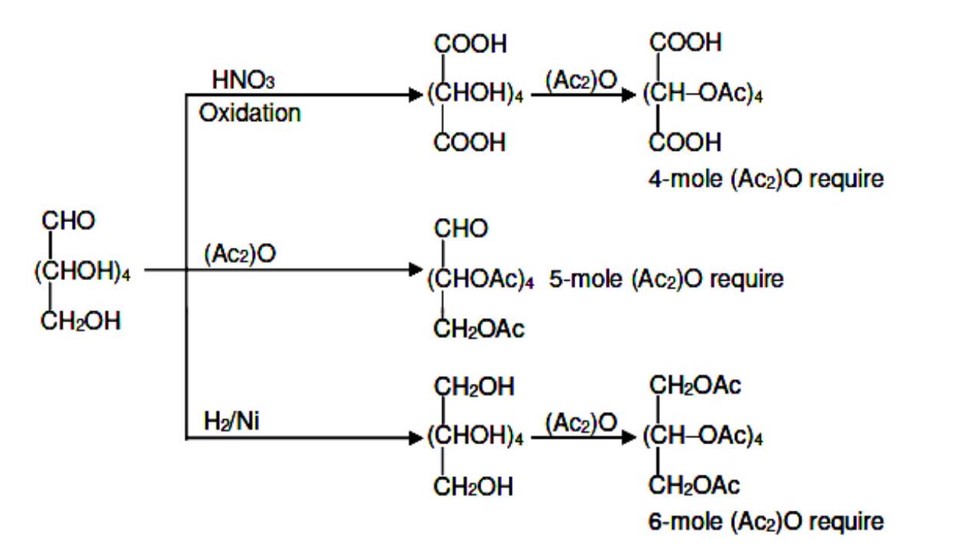
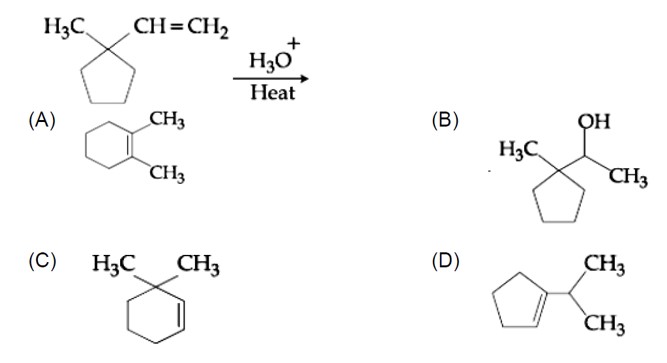
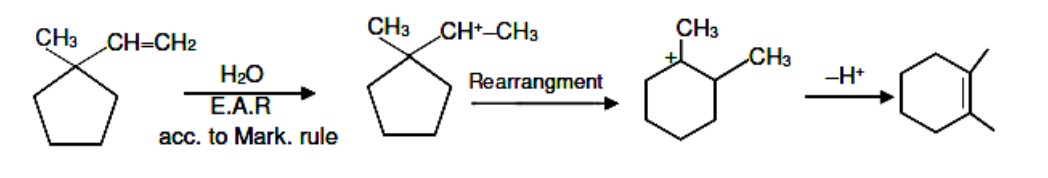
Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 9
With weak field ligands Mn (II) will be of high spin and with strong field ligands it will be of low spin.
Ni (II) tetrahedral complexes will be generally of high spin due to sp³ hybridisation. Mn (II) is of light pink color in aqueous solution.
New answer posted
4 months agoContributor-Level 9
On moving Left to Right along a period.
Atomic Radius → decreases.
Electronegativity → Increases.
Electron gain enthalpy → Increases.
Ionisation Enthalpy → Increases
New answer posted
4 months agoContributor-Level 9
The vapour pressure of solution will be less than vapour pressure of pure solvent, so some vapour molecules will get condensed to maintain new equilibrium.
New answer posted
4 months agoContributor-Level 9
Bredig's Arc Method is used for preparation of colloidal sol's of less reactive metal like Au, Ag, Pt.
New answer posted
4 months agoContributor-Level 9
Since spin only magnetic moment is 4.90BM so number of unpaired electrons must be 4, so If the complex is octahedral, then it has to be high spin complex with configuration t? g? e²g¹ in that case
CFSE = 4* (-0.4Δ? ) + 2*0.6Δ? = -0.4Δ?
If the complex is tetrahedral then its electronic configuration will be e? ², t? g² and CFSE will be = 3* (-0.6Δ? ) + 3* (0.4Δ? ) = -0.6Δ?
New answer posted
4 months agoContributor-Level 9
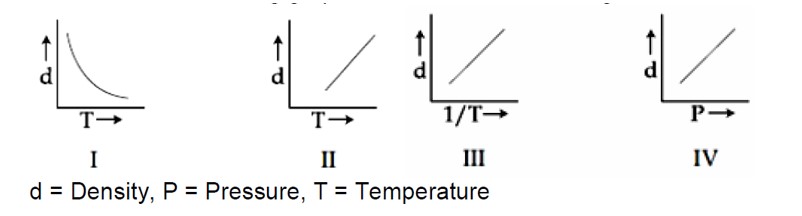
For ideal gas
PM = dRT
d = [PM]/R * 1/T
So graph between dV ST is not straight line
New answer posted
4 months agoContributor-Level 9
In this acid base Titrating there is no use of Bunsen burner and measuring cylinder other laboratory equipments will be required for getting the end point of titration.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers