Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The existence of atomic spectra tells us that energy levels in atoms are quantised. When atoms absorb or emit light, they do so at specific wavelengths. They lead to line spectra instead of a continuous spectrum. Now, every line corresponds to an electron that transitions between fixed energy levels. This is to make the photon's energy equal to the difference between them. If energy levels were not discrete, the spectra would be continuous. So, the line spectra provide direct evidence that electrons in atoms occupy quantised energy states.
New answer posted
4 months agoContributor-Level 10
Metal of group 7, 8, & 9 dose not form interstitial hydride this is called hydride gap.
Mn → group - 7
Fe → group - 8
Co → group - 9
So, Cr will forms interstitial hydride.
New answer posted
4 months agoContributor-Level 10
Conjugate base is highly stable.
Acidic strength ∝ stability of conjugate base.
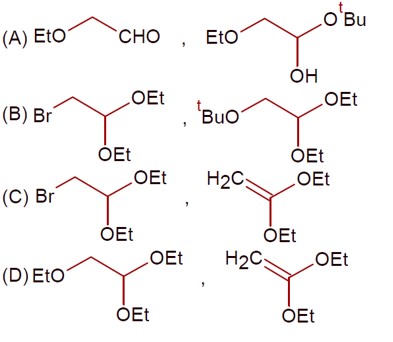
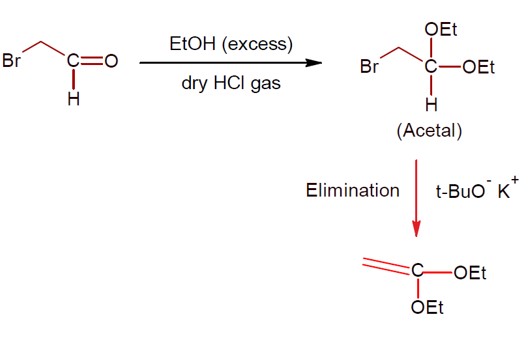
So, 
New answer posted
4 months agoContributor-Level 10
Phase | Medium | Colloid Type |
| (1) Cheese | liquid | solid | Gel |
| (2) Pumice stone | gas | solid | Solid sol |
| (3) Hair cream | liquid | liquid | Emulsion |
| (4) Cloud | liquid | gas | Aerosol |
New answer posted
4 months agoContributor-Level 10
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px¹ = π2py¹
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px¹
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px² = π2py¹
O? ²? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px² = π*2py²
B.O = (Bonding e? - Antibonding e? )/2
B.O of O? = (10 - 6)/2 = 2
B.O of O? = (10 - 5)/2 = 2.5
B.O of O? = (10 - 7)/2 = 1.5
B.O of O? ²? = (10 - 8)/2 = 1
New answer posted
4 months agoContributor-Level 10
Li? CO? decomposes easily on heating as;
Li? CO? - (Δ)-> Li? O + CO? ↑
NaHCO? is used in dry fire extinguishers.
K is most abundant element in cell fluid.
CsI is least soluble due to smaller hydration energy of Cs? & I?
New answer posted
4 months agoContributor-Level 10
Chelation increases stability of complex. Also more the chelation more the stability. Here, [Co (en)? (NH? )? ]Cl? , [Co (en)? ]Cl? and [Co (en) (NH? )? ]Cl? have chelation due to ethylenediamine. Therefore [Co (en)? ]Cl? has the highest chelation so the highest stability.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers