Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Using, µ = √n (n+2) B.M, n = number of unpaired electrons.
? Ti? ³ = 4s? 3d¹, unpaired electron = 1
µ = 1.73 B.M
? V? ² = 4s? 3d³, unpaired electron = 3
µ = 3.87 B.M
? Sc? ³ = 4s? 3d? , unpaired electron = 0
µ = 0 B.M
New answer posted
4 months agoContributor-Level 10
Anions have larger radii than atoms. Also, higher the e/p ratio higher the ionic radii. So, N? ³ > O? ² > F?
New answer posted
4 months agoContributor-Level 10
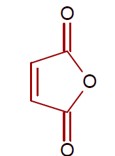
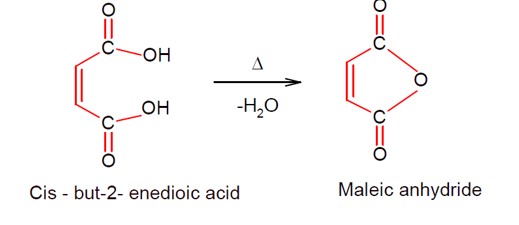
Heating of cis-but-2-enedioic acid gives maleic anhydride as shown.
[Chemical reaction showing conversion of cis-but-2-enedioic acid to maleic anhydride upon heating]
New answer posted
4 months agoContributor-Level 10
CFC breakdown by visible light to give Cl radical which react with stratospheric ozone.
CFC, CF? Cl? - (hv)-> Cl• + •CF? Cl
Cl• (g) + O? → ClO• + O?
ClO• + O → Cl• + O?
Atmospheric ozone reacts with NO to give NO? and O?
O? + NO → NO? + O?
New answer posted
4 months agoContributor-Level 10
Bohr's model solved the instability problem by proving about stationary states. In such stats, the electrons move in fixed orbits. They do not emit energy. This contradicted classical electromagnetic theory of Maxwell, which says accelerating charges should emit radiation and collapse into the nucleus. Bohr simply assumed Maxwell's laws don't apply to these special orbits.
New answer posted
4 months agoContributor-Level 10
Bohr's model is too simple for atoms beyond hydrogen. In multi-electron atoms like helium, it fails because it ignores a couple of aspects. First is the electron-to-electron repulsion, and second is the shielding effect, where inner electrons reduce the nuclear pull on outer ones. Due to both, orbitals with the same principal quantum number don't have the same energy. Bohr's model of atom assumes that it should have the same energy.
New answer posted
4 months agoContributor-Level 10
For a reaction to be spontaneous;
So, minimum T at which reaction will be spontaneous is 200 K.
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Moles of SO2 =
=0.01 mole
Moles of NaOH = 0.1 * 0.1
= 0.01 mole
SO2+NaOHNaHSO3
0.01 mole0.01 mole-
-0.01 mole
Non-volatile solute is NaHSO3
Moles of water =
Using ; relative lowering in V.P
Where; is lowering in V.P
i for NaHSO3 = 2
here; since solution is dilute
So; x = 24
New answer posted
4 months agoContributor-Level 10
PV = nRT
1 * V =
V = 2.4 litre
Vol of O2 adsorbed per gm = 2.4 / 1.2 = 2 litre
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers