Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
SF4 has sp3d hybridization and one type is axial while other type is equatorial
SiF4 has sp3 (all bonds are equal)
has sp3 (all bonds are equal)
XeF4 has sp3d2 and shape is sq. planar (All bonds are equal)
New answer posted
4 months agoContributor-Level 10
Viscosity of the hydrophilic sol is always higher than H2O, because dispersed phase particle attracted by dispersion medium.
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
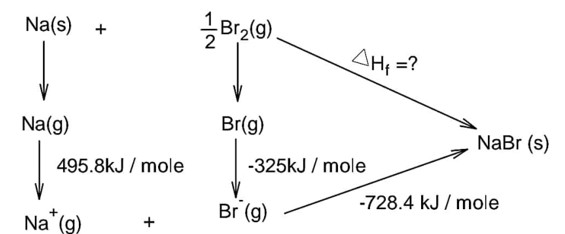
Bond dissociation energy of Br2 = 228 kJ/mole
Note : In question is neglect
New answer posted
4 months agoContributor-Level 10
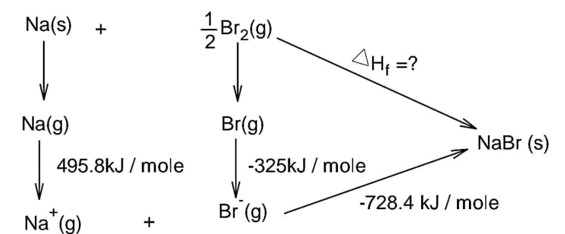
Bond dissociation energy of Br2 = 228 kJ/mole
Note : In question is neglected
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers