Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
(A) Isothermal process Temperature is constant throughout the process
(B) Isochoric process Volume is constant throughout the process
(C) Isobaric process Pressure is constant throughout the process
(D) Adiabatic process No exchange of heat between system and surrounding
New answer posted
4 months agoContributor-Level 10
(1) 4 mol of atoms
(2) 4 u of atom
(3) 4 g of Helium mole mole He atom
(4) of He at STP mole
New answer posted
4 months agoContributor-Level 10
(1) 4 mol of atoms
(2) 4 u of atom
(3) 4 g of Helium mole mole He atom
(4) of He at STP mole
New answer posted
4 months agoContributor-Level 10
At a given time is to be calculated and been compared with .
As , so reaction has a tendency to move backward.
New answer posted
4 months agoContributor-Level 10
Nucleophilic addition of sodium hydrogen sulphite to aldehyde or ketone is a;
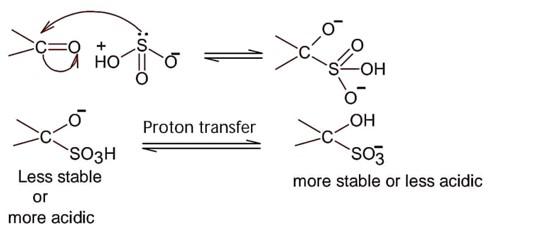
So; nucleopilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
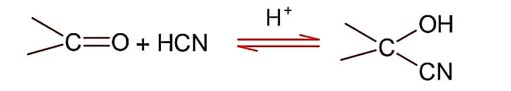
Addition of hydrogen cyanide;
Final product is cyanohydrin.
New answer posted
4 months agoContributor-Level 10
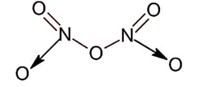
All given oxide have nitrogen – nitrogen bond except N2O5 as ;
New answer posted
4 months agoContributor-Level 10
BOD is biological oxygen demand which represents the amount of oxygen required to degrade organic matter in water.
Higher the BOD more polluted the water is
So; here water sample with BOD = 3 ppm is the cleanest
New answer posted
4 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers