Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
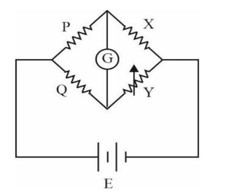
Resistance of P & Q should be approximately equal as it decreases error in experiment.
New answer posted
3 months agoContributor-Level 10
At different positions -NO? affects acidic strength differently. The order of acidic strength is p-nitrophenol > o-nitrophenol > m-nitrophenol.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
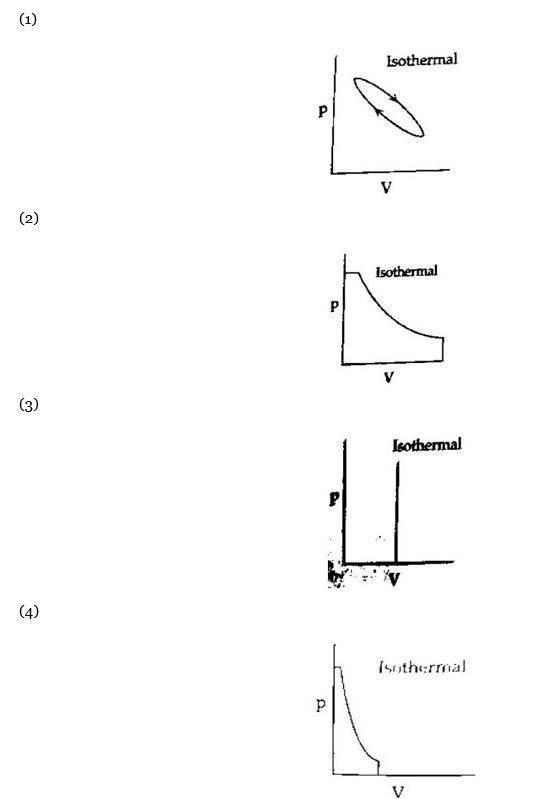
Maximum work is done in the case where area under the curve is maximum.
New answer posted
3 months agoContributor-Level 10
Distance in sec. i.e. to
Distance in sec.
i.e. to
So as we know
acceleration
New answer posted
3 months agoContributor-Level 10
O? (15) will have configuration σ1s²σ1s²σ2s²σ2s²σ2p? ² (π2p? ²=π2p? ²) (π*2p? ¹). This ion is paramagnetic.
New answer posted
3 months agoContributor-Level 10
Gadolinium has outer configuration of [Xe]4f?5d¹6s².
Its third ionization energy is low due to highly exchange energy and hence stability of the half-filled f subshell.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers