Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
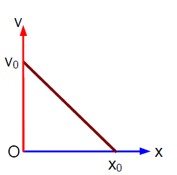
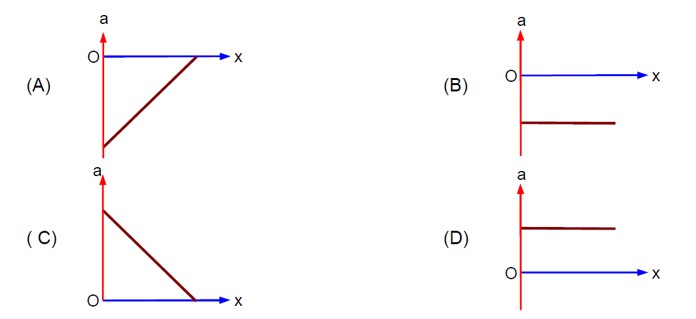
(v/v? ) + (x/x? ) = 1 ⇒ v = - (v? /x? )x + v?
⇒ a = dv/dt = - (v? /x? ) (v) = - (v? /x? ) [- (v? /x? )x + v? ] ⇒ a = (v? ²/x? ²)x - v? ²/x?
- Concept involved: Graph of kinematics
- Topic: Kinematics
- Difficulty level: Moderate
- Note: IIT-Jee-2005
- Point of Error: Writing Equation of straight line and differentiation
New answer posted
4 months agoContributor-Level 10
Limit (θ→0) [tan(πcos²θ) / sin(2πsin²θ)]
Let θ → 0. Then cos²θ → 1 and sin²θ → 0.
Let u = πsin²θ. As θ → 0, u → 0.
cos²θ = 1 - sin²θ = 1 - u/π.
The expression becomes:
Limit (u→0) [tan(π(1 - u/π)) / sin(2u)]
= Limit (u→0) [tan(π - u) / sin(2u)]
= Limit (u→0) [-tan(u) / sin(2u)]
= Limit (u→0) [-tan(u) / (2sin(u)cos(u))]
= Limit (u→0) [-(sin(u)/cos(u)) / (2sin(u)cos(u))]
= Limit (u→0) [-1 / (2cos²(u))] = -1 / (2 * 1²) = -1/2.
New answer posted
4 months agoContributor-Level 9
Combustion of ethane: C? H? + 7/2 O? → 2CO? + 3H? O.
Moles of C? H? = 3g / 30 g/mol = 0.1 mol.
Moles of H? O produced = 0.3 mol.
Number of H? O molecules = 0.3 * 6.023 * 10²³ ≈ 18.06 * 10²².
So, x = 18.
New answer posted
4 months agoContributor-Level 10
P(O at even place) = 1/2, P('O' at odd place) = 1/3
P(1 at even place) = 1 - 1/2 = 1/2
P(1 at odd place) = 1 - 1/3 = 2/3
The probability that 10 is followed by 01 is given by a sequence of probabilities, which seems to represent a specific arrangement or transition. The calculation is:
P(10 is followed by 01) = (2/3) * (1/2) * (1/3) * (1/2) * (1/2) * (1/3) * (1/2) * (2/3) = 1/18 + 1/18 = 1/9.
The calculation seems incomplete or context is missing.
New answer posted
4 months agoContributor-Level 9
3-Hydroxy propanal
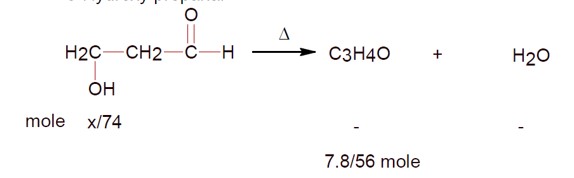
If 7.8g of C? H? O (molar mass 56 g/mol ) is formed, calculate the initial weight of 3-hydroxy propanal (molar mass 74 g/mol ).
Weight = (7.8/56) * 74 * (100/64) [Assuming 64% yield, though the number seems out of place].
Ans ≈ 16 g.
New answer posted
4 months agoContributor-Level 9
AX is a diatomic molecule with a bond order of 2.5.
The compound is NO. The total number of electrons = 15 (7+8).
New answer posted
4 months agoContributor-Level 10
- Given vectors OP = xi + yj - k and OQ = -i + 2j + 3xk.
- PQ = OQ - OP = (-1 - x)i + (2 - y)j + (3x + 1)k
- Given |PQ| = √20, so |PQ|² = 20.
(-1 - x)² + (2 - y)² + (3x + 1)² = 20
(1 + x)² + (2 - y)² + (3x + 1)² = 20 .(i) - Given OP ⊥ OQ, so OP · OQ = 0.
(x)(-1) + (y)(2) + (-1)(3x) = 0
-x + 2y - 3x = 0 ⇒ -4x + 2y = 0 ⇒ y = 2x .(ii)
Substitute (ii) into (i):
(1 + x)² + (2 - 2x)² + (3x + 1)² = 20
1 + 2x + x² + 4 - 8x + 4x² + 9x² + 6x + 1 = 20
14x² = 14 ⇒ x² = 1 ⇒ x = ±1.
When x = 1, y = 2. When x = -1, y = -2.
So, (x, y) can be (1, 2) or (-1, -2).- Given that OR, OP, and OQ are coplanar, their scalar triple product is 0: [OR OP OQ]
- Given OP ⊥ OQ, so OP · OQ = 0.
New answer posted
4 months agoContributor-Level 9
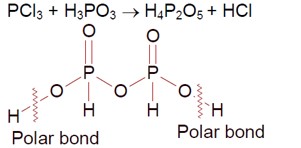
Phosphorous Trichloride + Phosphorous acid
O-H bond is polar, so the number of ionisable hydrogens are 2.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers