Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Given the determinant:
| α β γ |
| β γ α | = 0
| γ α β |
The expansion of this determinant is - (α³ + β³ + γ³ - 3αβγ) = 0.
This implies (α+β+γ) (α²+β²+γ²-αβ-βγ-γα) = 0.
From a cubic equation x³ + ax² + bx + c = 0 with roots α, β, γ:
α+β+γ = -a
αβ+βγ+γα = b
αβγ = -c
Substituting into the determinant condition:
(-a) ( (α+β+γ)² - 3 (αβ+βγ+γα) ) = 0
(-a) ( (-a)² - 3b ) = 0
-a (a² - 3b) = 0
a (a² - 3b) = 0
This implies a=0 or a²=3b. If a≠0, then a²=3b, so a²/b = 3.
New answer posted
4 months agoContributor-Level 10
Given the family of parabolas y² = 4a (x+a).
Differentiate with respect to x:
2y (dy/dx) = 4a
a = (y/2) (dy/dx)
Substitute a back into the original equation:
y² = 4 * (y/2) (dy/dx) * [x + (y/2) (dy/dx)]
y² = 2y (dy/dx) * [x + (y/2) (dy/dx)]
y = 2 (dy/dx) * [x + (y/2) (dy/dx)]
y = 2x (dy/dx) + y (dy/dx)²
y (dy/dx)² + 2x (dy/dx) - y = 0
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
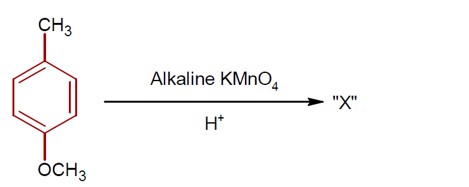
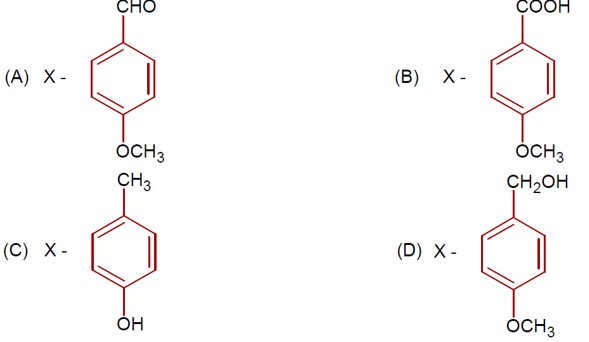
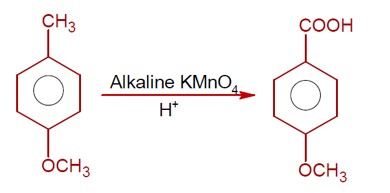
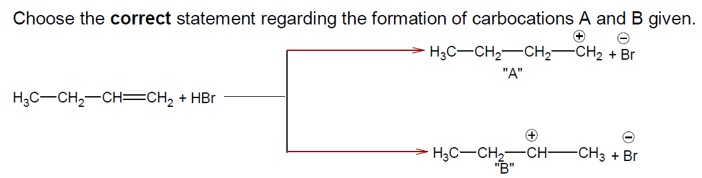
The addition of HBr to H? C−CH? −CH=CH? proceeds via carbocation formation.
Formation of a 1° Carbocation (A): H? C−CH?
Formation of a 2° Carbocation (B): H? C−CH? −C? H−CH?
The 2° carbocation (B) is more stable than the 1° carbocation (A). Therefore, the activation energy (Ea) for the formation of B is lower, and B is formed faster.
New answer posted
4 months agoContributor-Level 9
o Alcoholic potassium hydroxide (Alc KOH):- used for β - elimination.
o Pd / BaSO? : - Lindlar's Catalyst.
New answer posted
4 months agoContributor-Level 10
A diagonal relationship is observed in the periodic table between elements of period (2) and period (3).
Li and Mg
Be and Al
B and Si
Li and Na do not show a diagonal relationship.
New answer posted
4 months agoContributor-Level 10
For the coagulation of a negative sol, the species Ba²? has the highest flocculating power (referring to the Hardy-Schulze rule, where higher charge leads to greater coagulation power).
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 9
Quantum Numbers and Orbitals:
o Total Node = n - 1 (l => Azimuthal Q.N)
o Radial Node = n - l - 1
o Angular Node: l
o If Angular node = 0, then l = 0, i.e., S orbital.
o If Radial nodes = 2, then n - l - 1 = 2.
o Substituting l = 0 gives n - 0 - 1 = 2, so n = 3.
o Therefore, the orbital is 3s.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers