Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 9
While the particle moves from mean position to displacement, half of its amplitude, its phase changes by π/6 rad. So,
Time taken, t = (π/6)/ω = T/12 = (2/12)s = (1/6)s
a = 6
New answer posted
4 months agoContributor-Level 9
Using Conservation of Mechanical Energy at point-A and at point-B, we can write
K_B = U_A - U_B [Since K_A = 0]
⇒ (1/2)mv_B² = mg (h_A - h_B)
⇒ v_B = √ (2 * 10 * (10 - 5) = 10m/s
New answer posted
4 months agoContributor-Level 9
For Wire-A
F / (πr_A²) = Y (l_A / 2) . (1)
For Wire-B
F / (πr_B²) = Y (l_B / 4) . (2)
From equations (1) and (2), we can write
(l_A / (2r_A²) = (l_B / (4r_B²) ⇒ l_B/l_A = 2 (r_B/r_A)² ⇒ x = l_B = 32
New answer posted
4 months agoContributor-Level 9
Angle from direction of flow = 90° + θ = 120°
⇒ θ = 30°
sin 30° = u/v ⇒ u/10 = 1/2 ⇒ u = 5 m/s
New answer posted
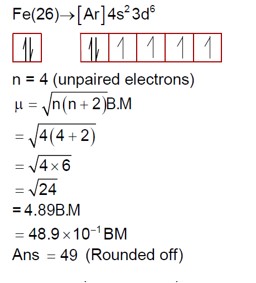
4 months agoContributor-Level 10
The reaction is: FeCl? + 3H? C? O? + 3KOH → K? [Fe (C? O? )? ] (A) + 3HCl + 3H? O
In the complex [Fe (C? O? )? ]³? , the oxalate ion (C? O? )²? is a bidentate ligand.
There are three bidentate ligands, so the coordination number, or secondary valency, of Fe is 3 * 2 = 6.
New answer posted
4 months agoContributor-Level 10
The cell constant (G) is given by G = κ * R, where κ is conductivity and R is resistance.
Since the cell constant is constant: (κ * R)KCl = (κ * R)HCl
0.14 Sm? ¹ * 4.19 Ω = κ_HCl * 1.03 Ω
κ_HCl = (0.14 * 4.19) / 1.03 = 0.569 Sm? ¹
This is equivalent to 56.9 * 10? ² Sm? ¹.
The answer, rounded off, is 57.
New answer posted
4 months agoContributor-Level 9
Apply conservation of momentum along y-axis, we can write
10v? - 10v? sin 30° = 0
⇒ v? = 20m/s
New answer posted
4 months agoContributor-Level 9
Deceleration, a = u² / 2S = 10² / (2 * 0.5) = 100 m/s².
Retarding force, F = MA = 0.1 * 100 = 10N
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers