Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
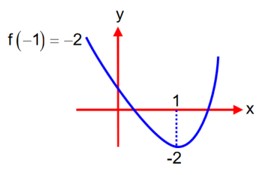
If
Y = 3K (1 - 2
and Y = 2
With the help of equations (1) and (2), we can write
New answer posted
4 months agoContributor-Level 10
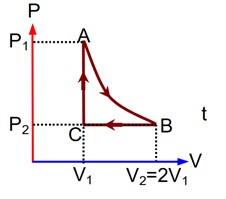
In isothermal process, temperature is constant.
In isochoric process, volume is constant.
In adiabatic process, there is no exchange of heat.
In isobaric process, pressure is constant
New answer posted
4 months agoContributor-Level 10
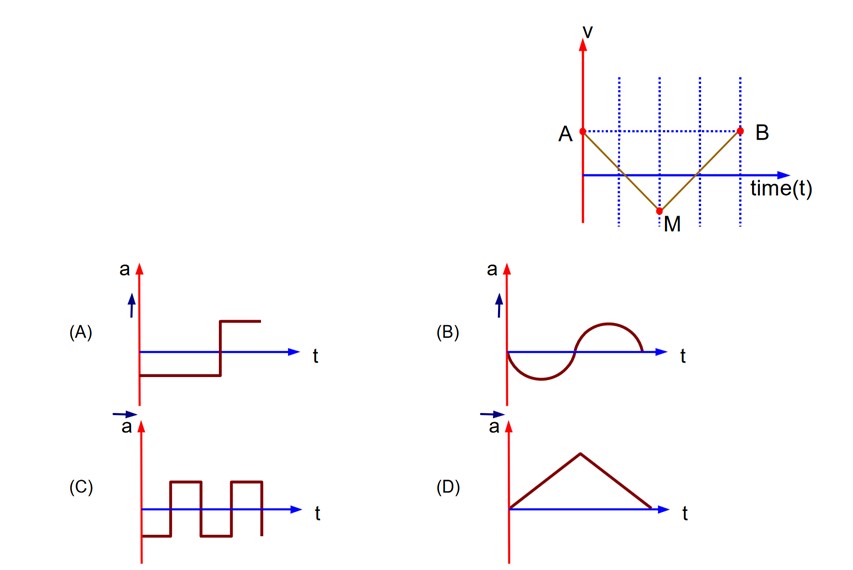
For part AM, slope of v – t graph is constant but negative. For part MB, slope of v – t graph is constant but positive.
New answer posted
4 months agoContributor-Level 10
For a reaction to be spontaneous;
So, minimum T at which reaction will be spontaneous is 200 K.
New answer posted
4 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers