Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Polarisation power µ for K+, polarising power is least and ionic character is maximum.
New answer posted
3 months agoNew answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
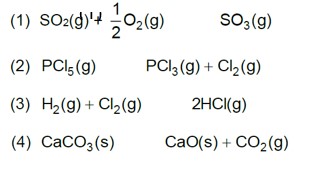
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
New answer posted
3 months agoContributor-Level 10
We consider the enthalpy change (delta H) more important than the internal energy (delta U) for practical reasons. When we go by the definitions alone, internal energy is the heat absorbed or released at a constant volume. This only happens in a sealed container. But in real life experiments held at laboratories, we have open vessels like test tubes, and there, only atmospheric pressure as a state variable remains constant, and not volume per se. This condition of constant pressure becomes more useful to calculate enthalpy in the chemical reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers