Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
4.21. According to VSEPR theory, if CH4 were square planar, the bond angle would be 90°. For tetrahedral structure, the bond angle is 109°28? Therefore, in square planar structure, repulsion between bond pairs would be more and thus the stability will be less.
Moreover, the orbitals of carbon in ground and excited states look as shown below. In the excited state, the electrons from 4 H atoms occupy one 2s orbital and three 2p orbitals. This results in an sp3 hybridisation, which is the hybridisation of a tetrahedral geometry

New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
4.19. N2 < SO2 < ClF3 < K2O
The ionic character in a molecule is dependent upon the electronegativity difference between the constituting atoms. The greater the difference, the greater will be the ionic character of the molecule.
New answer posted
8 months agoContributor-Level 10
4.18. When two dissimilar atoms having different electronegativities combine to form a covalent bond, the bond pair of electrons is not shared equally. The bond pair shifts towards the nucleus of the atom having greater electronegativity. As a result, electron distribution gets distorted and the electron cloud is displaced towards the electronegative atom.
As a result, the electronegative atom becomes slightly negatively charged while the other atom becomes slightly positively charged. Thus, opposite poles are developed in the molecule and this type of a bond is called a polar covalent bond.
For example, in hydrogen fluoride molecule (HF
New answer posted
8 months agoContributor-Level 10
4.17. Electronegativity: Electronegativity is the tendency of an atom to attract shared pair of electrons towards itself. Electronegativity of any given element is never the same.
It depends on the element it is bonded with in a compound. Electronegativity is a relative quantity and cannot be measured.
Whereas, electron gain enthalpy is the change in enthalpy when an electron is added to a neutral gaseous atom to form an anionic specie.
It can have a positive or negative value. Every element has a specific electron gain enthalpy value.
New answer posted
8 months agoContributor-Level 10
4.16. Significance of dipole moment are:
In predicting the nature of the molecules: Molecules with specific dipole moments are polar in nature and those of zero dipole moments are non-polar in nature.
In the determination of shapes of molecules.
In calculating the percentage ionic character.
New answer posted
8 months agoContributor-Level 10
4.15. In CO2, there are two C=O bonds. Each C=O bond is a polar bond. The net dipole moment of CO2 molecule is zero. This is possible only if CO2 is a linear molecule. (O=C=O). The bond dipoles of two C=O bonds cancel the moment of each other.

Whereas, H2O has a net dipole moment of 1.84 D. H2O molecule has a bent structure because here the O—H bonds are oriented at an angle of 104.5° and do not cancel the bond moments of each other.

New answer posted
8 months agoContributor-Level 10
velocity of a freely falling body is v=
And
-1
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly
a) 1.2 nm (b) 1.2 x 10-3 nm
(c) 1.2 x 10-6 nm (d). 1.2 x 10 nm
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
(d)
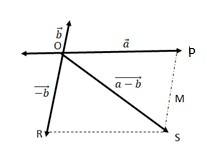
We can write
OS + PS > OP …….(i)
OS > PS – OP …….(ii)
+ ….(iii)
The quantity on the LHS is always positive and that on the RHS can be positive or negative. To make both quantities positive, we take modulus of both sides as:
> ….(iv)
If the two vectors and act along a straight line but in the opposite direction, then we can write = -
Combining (iv) and (v), we get
-
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers


