Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
13. In ccp lattice the number atoms per unit cell = 4
The number of tetrahedral voids is given as = 2 (n) = 2 x 4 = 8
Only one-third of tetrahedral voids are occupied by metal M so, the ratio of atoms of element M to that of element N = 1/3 (8) : (4) = 2 : 3
or M : N = 2 : 3
Hence, the formula of the compound is M2N3.
New answer posted
8 months agoContributor-Level 10
The given D.E. is
Which is of form.
So,
Thus the general solution is of form,
New answer posted
8 months agoContributor-Level 10
12. When tetravalent germanium is doped with trivalent gallium then some of the positions of the lattice of germanium gets occupied by the gallium. Since the gallium atom has only three valence electrons, the fourth valency of the nearby germanium atom does not get satisfied and hence this place remains vacant. This place is deficient of electrons and is therefore called an electron hole or electron vacancy.
Now, the electron from the neighbouring atom comes and fills the gap and leads to the formation of a hole in its original position. Under the influence of electric fields, the electrons move towards the positively charged plat
New answer posted
8 months agoContributor-Level 10
The given D.E is
Which is of form
So,
Thus the general solution is of the form,

New answer posted
8 months agoContributor-Level 10
The given D.E is

Which is of form
So,
Thus the solution is of the form.
New answer posted
8 months agoContributor-Level 10
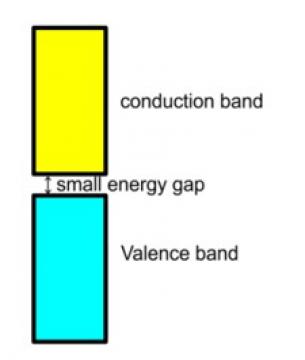
11. In semiconductors the gap between conduction band and valence band is small and hence some of the electrons from the valence band can easily jump to the conduction band and shows some conductivity but with rise in the temperature more of the electrons gets jump to the conduction band and thus, their electrical conductivity increases with rise in the temperature.
New answer posted
8 months agoContributor-Level 10
The given D.E. is
Which is of form
So ,
Thus the solution is of the form,
New answer posted
8 months agoContributor-Level 10
The given D.E. is
Which is of form
So,
Thus, the general solution is of the form
New answer posted
8 months agoContributor-Level 10
10. The ZnO crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at the interstitial sites and on heating this white crystal it loses oxygen and turns yellow. The reaction involved is given as-
ZnO Zn2+ + O2 + 2e-
Here the excess of Zn2+ ions move to the interstitial sites and electrons to neighbouring interstitial sites.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers