Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
44. Option (i) and (ii)
Explanation: Depressants are materials that are added for the separation of ores that prevent certain types of particles from coming to froth and forming bubbles. For example, an ore containing ZnS and PbS, NaCN is used as a depressant.
When sulfur ores are blown in hot air along with silica, the solidified metal which is obtained has a blistered appearance due to SO2 evolution.
New answer posted
8 months agoContributor-Level 10
25. The sum of sublimation energy and ionisation enthalpy to oxidise cu (s) to Cu2+ is so highly that it is not compensated by the hydration enthalpy of Cu. Due to this, the Eof Cu is positive.
While in case if Zn, the E value is negative or more negative than the expected value because when the electrons are removed from the 4s-orbital. Zn acquires a stable 3d10 configuration state.
New answer posted
8 months agoContributor-Level 10
The equation of the curve is - (1) and
lines are
- (2)
- (3)
- (4)
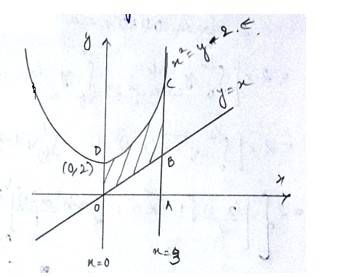
Equation (1)is a parabola with vertex (0,2)
Equation (2)is a straight line passing origin with shape =
The required area enclosed OBCDO = area (ODCAO)-area (OBAO)
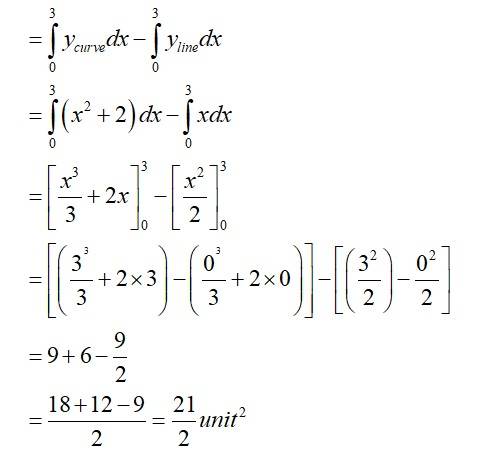
New answer posted
8 months agoContributor-Level 10
24. As an effect of lanthanide contraction, the second and third rows of transition elements resemble each other. For example, zirconium and hafnium have similar radius i.e. 160pm and 159pm respectively. Due to this similarity in their size, they show similar physical and chemical properties.
Lanthanoid contraction: Because the elements in Row 3 have 4f electrons. These electrons do not shield good, causing a greater nuclear charge. This greater nuclear charge has a greater pull on the electrons and result in the decrease in their size and atomic radii.
New answer posted
8 months agoContributor-Level 10
43. Option (i) and (ii)
In the vapor phase refining method, the metal is converted into its volatile compound and then is collected elsewhere. This involves two techniques:
1. Mond Process for refining Nickel: in this process, a volatile complex, nickel tetracarbonyl is formed when nickel is heated with a stream of carbon monoxide.
2. Van Arkel Method for refining Zirconium or Titanium: this method is basically used for removal of oxygen and nitrogen present as impurities in the Zr or Ti metal. These are heated in an evacuated metal with iodine. As iodine is more covalent than these metals, it volatilizes.
New answer posted
8 months agoContributor-Level 10
The equation of the given circle is
- (1)
- (1) - (2)
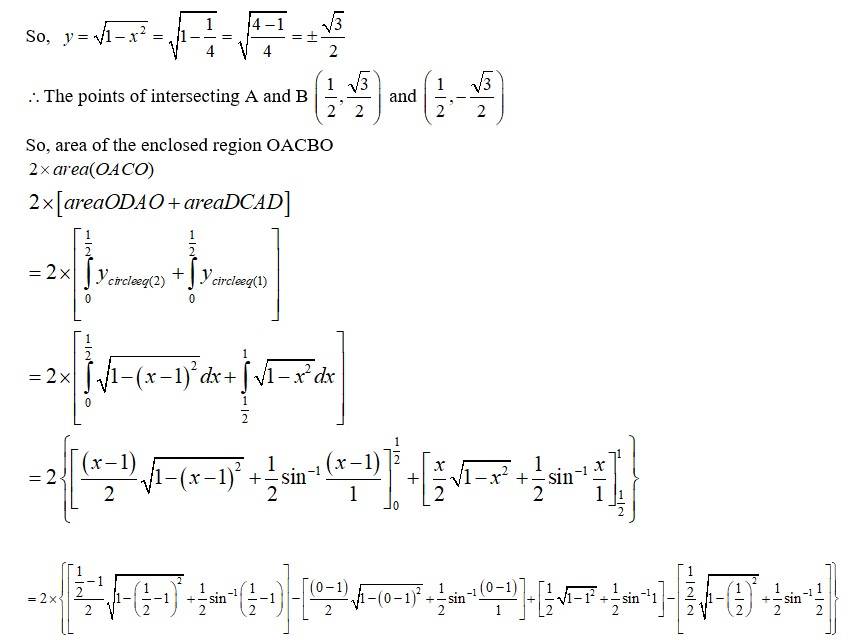
Equation (1) is a circle with centre 0 (0,0) and radius 1. Equation (2) is a circle with centre c (1,0) and radius 1.
Solving (1) and (2)
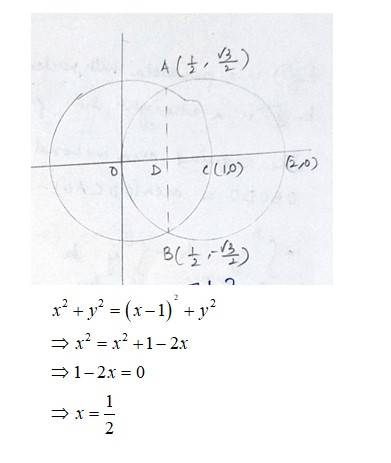
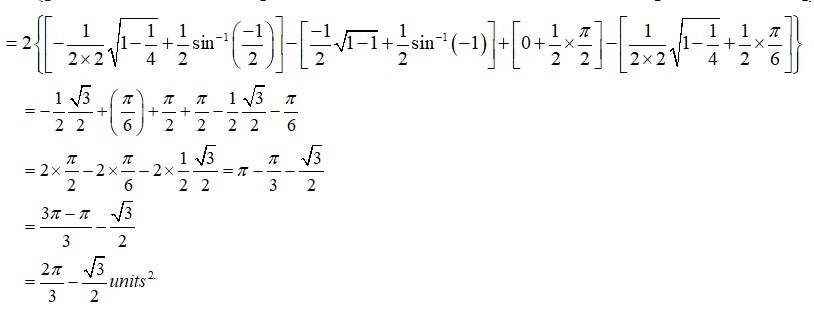
New answer posted
8 months agoContributor-Level 10
23. KMnO4 act as an oxidising agent however it's activity as oxidising agent is influenced by the pH of the solution i.e. acidic, basic or neutral solution.
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O
(Orange) (yellow)
2K2CrO4 + H2SO4 → K2Cr2O7 + K2SO4 + H2O
(yellow) (orange)
New answer posted
8 months agoContributor-Level 10
22. This is due to the inter conversion of dichromate (orange) to chromate ion (yellow).
Cr2O72- CrO42-
(orange) (yellow)
New answer posted
8 months agoContributor-Level 10
42. Option (i) and (iv)
(i) Carbon monoxide is the primary reducing agent in the furnace.
(ii) This is an endothermic reaction in which heat is absorbed from the furnace. As a result, it is critical not to add too much limestone, as this will cool the furnace. Calcium oxide is a basic oxide that reacts with acidic oxides in the rock, such as silicon dioxide. Calcium silicate is formed when calcium oxide reacts with silicon dioxide.
New answer posted
8 months agoContributor-Level 10
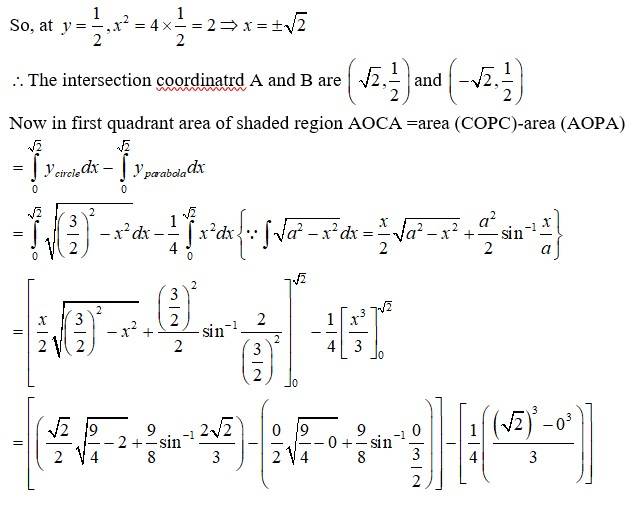
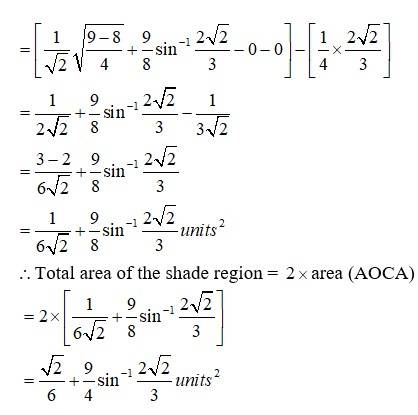
The equation given circle is
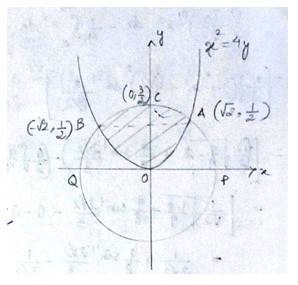
i.e, centre (0,0), radius
since intersect the circle
we can put in
which is not possible or cannot be (-)ve
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
