Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
A.
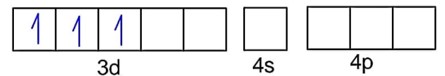
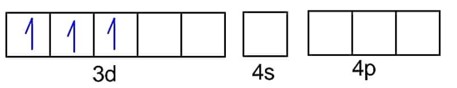
Fe3+ - 3d54s0
F- is weak field ligand, so no pairing of electrons :
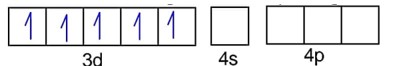
Number of unpaired electrons, n = 5
B.
CN- is strong field ligand, so pairing of electrons takes place.
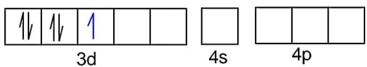
Number of unpaired electrons, n = 1
C.
New answer posted
6 months agoContributor-Level 10
Electronic configuration of the given ions is :
Hence, Eu2+ and Tb4+ have half | filled f-orbitals and Yb2+ have completely filled f-orbitals.
New answer posted
6 months agoContributor-Level 10
In 3d-series, all metals except Cu have negative value of
New answer posted
6 months agoContributor-Level 10
Fluorine forms only one oxoacid which is hypofluorous acid HOF because it shows only – 1 oxidation state. Which is due to its the smallest size among halogens & the highest electronegativity.
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
Higher the E.N. difference between hydrogen and other atom then higher be the strength of intermolecular H-bond
Here, order of difference in E.N is
O - H > N – H > C - H
Hence, correct order of H bond strength is,
CH4 < HCN < NH3
New answer posted
6 months agoContributor-Level 10
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
New answer posted
6 months agoContributor-Level 10
Structure PCl5 is trigonal bipyramidal,
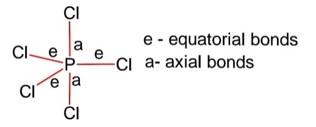
Hybridization of P is sp3d
Equatorial bonds lie in a plane
Axial bonds are longer than equatorial bonds so axial bonds are weaker than equatorial bonds.
New answer posted
6 months agoContributor-Level 10
Fluorine forms only one oxoacid which is hypofluorous acid HOF because it shows only – 1 oxidation state. Which is due to its the smallest size among halogens & the highest electronegativity.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers