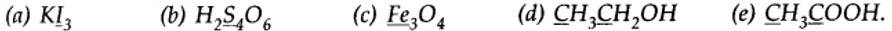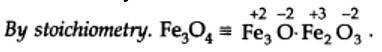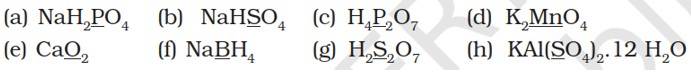Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
(a) In Kl3, since the oxidation number of K is +1, therefore, the average oxidation number of iodine = -1/3. But the oxidation number cannot be fractional. Therefore, we must consider its structure, K+ [I —I < I]–. Here, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine atoms forming the I2 molecule is zero, while that of iodine forming the coordinate bond is -1. Thus, the oxidation number of the three I atoms, atoms in Kl3 is 0, 0 and -1, respectively.
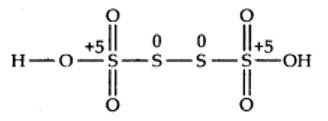
(b) By conventional method O.N. of S in H2S4O6is calculated as:
2 (+1) +4x + 6) (-2) = 0
Or x = +2.5
But all the four
New answer posted
6 months agoContributor-Level 10
Let x be the oxidation number to the underlined elements in the given species:
(a) NaH2PO4
(+1) + 2 (+1) + x + 4 (-2) = 0
x + 3 – 8 = 0
x = +5
(b) NaHSO4
(+1) + (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(c) H4P2O7
4 (+1) + 2x + 7 (-2) = 0
2x -10 =0
x = +5
(d) K2MnO4
2 (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(e) CaO2
2 + 2x = 0
x = -1
(f) NaBH4
1 + x + 4 (-1) = 0 (Since H is present as hydride ion.)
x = +3
(g) H2S2O7
2 (+1) + 2x + 7 (-2) = 0
x = +6
(h) KAl (SO4)2.12H2O
+1 + 3 + 2x + 8 (-2) + 12 (2 x 1 - 2) = 0
x = +6
New answer posted
6 months agoContributor-Level 10
5.18. Given, P1 = P2 and V1 = V2
We know that P1V1 = P2V2
Or, n1RT1 = n2RT2
i.e., n1T1 = n2T2
Substituting n = w/M, we get
(W1/M1) x T1 = (W2/M2) x T2
(2.9/M1) x (95 + 273) = (0.184/2) x (17 + 273)
M1 = (2.9 x 368 x 2) / (0.184 x 290) = 40 g mol-1
New answer posted
6 months agoContributor-Level 10
5.17. No. of moles of CO2 = Given mass of CO2 / Molar mass
= 8.8g / 44g mol-1 = 0.2 mol
Pressure of CO2 = 1 bar
RR = 0.083 bar dm3 K–1 mol–1
T = 273 + 31.1 K = 304.1 K
According to ideal gas equation,
PV = nRT
Therefore, V = nRT/P
= (0.2 x 0.083 x 304.1) / 1 bar = 5.048 L
New answer posted
6 months agoContributor-Level 10
5.16. Radius of the balloon = 10 m
Therefore, volume of the balloon = (4/3)? r3 = (4/3) x (22/7) x (10 m)3
= 4190.5 m3
Volume of He filled at 1.66 bar and 27 °C = 4290.5 m3
To calculate the mass of He,
PV = nRT = (w/M) RT, where M is molar mass of He i.e. 4 g per mole or 4 x 10-3 kg mol-1
=> w = [ (4 x 10-3 kg mol-1) (1.66 bar) (4190.5 m3)] / [ (0.083 bar dm3 K-1 mol-1) (300K)]
= 1117.5 kg
Total mass of the balloon along with He = 100 + 1117.5 = 1217.5 kg
Maximum mass of the air that can be displaced by balloon to go up = volume x density
= 4190.5 m3 x 1.2 kg m-3 = 5028.6 kg
New answer posted
6 months agoContributor-Level 10
5.15. Molar mass of O2 = 32 g/mol
It means, 8 g of O2 has 8/32 mol = 0.25 mol
Molar mass of H2 = 2 g/mol
It means, 4 g of H2 has 4/2 mol = 2 mol
Therefore, total number of moles, n = 2 + 0.25 = 2.25 mol
Given, V = 1dm3, T = 27°C = 300 K, R = 0.083 bar dm3 K-1 mol-1
Applying PV = nRT,
P = nRT / V
= (2.25) (0.083 bar dm3 K-1 mol-1) (300 K) / (1dm3)
= 56.025 bar
New answer posted
6 months agoContributor-Level 10
5.14. Time taken to distribute 1010 wheat grains = 1s
Time taken to distribute Avogadro number of wheat grains = (1s x 6.022 x 1023) / 1010
= 6.022 x 1013 s
= (6.022 x 1013 / 60 x 60 x 24 x 365) year
= 1.9 x 106 years
New answer posted
6 months agoContributor-Level 10
5.13. Molecular mass of N2 = 28g
28 g of N2 has No. of molecules = 6.022 x 1023
1.4 g of N2 has No. of molecules = (6.022 x 1023 x 1.4 g)/28 g= 3.011 x 1022 molecules.
Atomic No. of Nitrogen (N) = 7
1 molecule of N2 has electrons = 7 x 2 = 14
3.011 x 1022 molecules of N2 have electrons= 14 x 3.011 x 1022= 4.215 x1023 electrons.
New answer posted
6 months agoContributor-Level 10
5.12. Given,
P= 3.32 bar
V= 5 dm3
n= 4 mol
R= 0.083 bar dm3 K-1 mol-1
PV = nRT
Or T = PV / nR = 3.32 x 5 dm3 / (4.0 mol x 0.083 bar dm3 K-1 mol-1)
= 50 K
New answer posted
6 months agoContributor-Level 10
9.56. Dihydrogen is prepared from water by the action of alkali metals like Na and K which is a strong reducing agent.
2Na + 2H2O → 2NaOH + H2
2K + 2H2O → 2KOH + H2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers