Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
ΔG° = + 25.2 kJ / mol
Using ΔG° = -2.3 RT log Kp
25.2 * 10³ = - 2.3 * 8.3 * 400 log Kp
o 3.3 = log Kp
log (1 / 2*10³) = log Kp
Kp = 1 / (2*10³)
Using; Kp = Kc (RT)
1 / (2*10³) = Kc (0.083 * 400)? ¹
New answer posted
4 months agoContributor-Level 10
Iron (III) iodide (FeI? ) does not exist because it is unstable. The Fe³? ion is a strong enough oxidizing agent to be easily reduced to Fe²? by the I? ion, which in turn is oxidized.
New answer posted
4 months agoContributor-Level 10
In sodium hydride (NaH), hydrogen has an oxidation state of -1. In this state, it can only act as a reducing agent.
New answer posted
4 months agoContributor-Level 9
Oxidation state of N
| NO | +2 |
|-|-|
| NO? | +4 |
| N? O | +1 |
| NO? | +5 |
So, order of oxidation state is
NO? > NO? > NO > N? O
New answer posted
4 months agoContributor-Level 9
Mn? O? is mixed oxide which contains MnO and Mn? O? , so Mn is in +2 and +3 oxidation state respectively. Both Mn? ² & Mn³? has unpaired electrons so Mn? O? will show magnetic property. While all other oxides have no unpaired electrons either on cation or on anion.
New answer posted
4 months agoContributor-Level 9
Be is used in X ray tube windows.
Mg is used in incendiary bombs & signals.
Compounds of Ca i.e CaCO? is used in extraction of metals like Fe.
Radium (Ra) is used in treatment of cancer in radiotherapy.
New answer posted
4 months agoContributor-Level 9
3-Hydroxy propanal
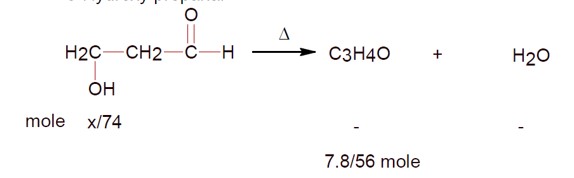
If 7.8g of C? H? O (molar mass 56 g/mol ) is formed, calculate the initial weight of 3-hydroxy propanal (molar mass 74 g/mol ).
Weight = (7.8/56) * 74 * (100/64) [Assuming 64% yield, though the number seems out of place].
Ans ≈ 16 g.
New answer posted
4 months agoContributor-Level 9
AX is a diatomic molecule with a bond order of 2.5.
The compound is NO. The total number of electrons = 15 (7+8).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers

