Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The maximum temperature that can be achieved in blast furnace is upto ![]() .
.
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
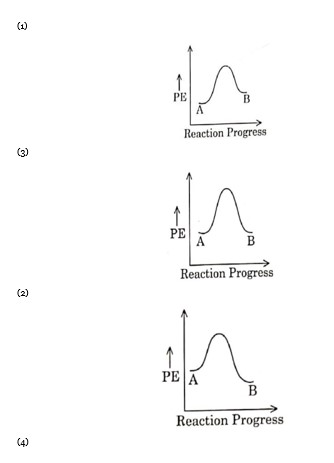
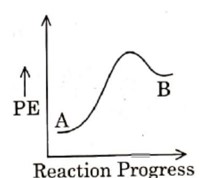
For a given reaction? H is negative. Hence, potential energy profile is of an exothermic reaction.
New answer posted
4 months agoContributor-Level 10
Due to lanthanoid contraction Zr and Hf has similar atomic and ionic radii.
New answer posted
4 months agoContributor-Level 10
No. of atoms in Hexagonal primitive unit cell = 6
No. of Tetrahedral voids = 2 * No. of atoms per unit cell
= 2 * 6 = 12
No. of Octahedral voids = No. of atoms per unit cell = 6
New answer posted
4 months agoContributor-Level 10
Pollution due to oxides of sulphur gets enhanced due to hydrocarbons and oxidizing agents like O? & H? O?
New answer posted
4 months agoContributor-Level 10
M = (a³ * d * N_A) / Z = (3.608 * 10? )³ * 8.92 * 6.022 * 10²³) / 4
M = (46.96 * 10? ²? * 8.92 * 6.022 * 10²³) / 4 = 63 g/mole
the closest answer is choice (1)
P = (nRT) / V = (2 * 0.0831 * 300) / 10 = 4.986 bar
New answer posted
4 months agoContributor-Level 10
Moles of HCl = (50 / 1000) * 0.5 = 0.025 moles
So, moles of CaCO? used = 0.025 / 2 = 0.0125 moles = 1.25 g
95% (Total mass of CaCO? ) = 1.25 g
Total mass of CaCO? = 1.25 / 0.95 = 1.32 g
New answer posted
4 months agoContributor-Level 10
p? = p? x? is not a correct form of Dalton's law of partial pressures.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers