Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
At different positions -NO? affects acidic strength differently. The order of acidic strength is p-nitrophenol > o-nitrophenol > m-nitrophenol.
New question posted
4 months agoNew answer posted
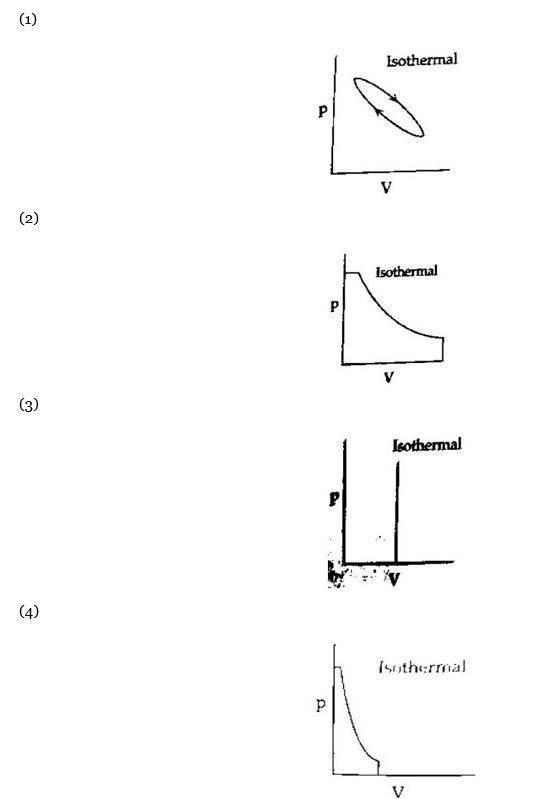
4 months agoContributor-Level 10
Maximum work is done in the case where area under the curve is maximum.
New answer posted
4 months agoContributor-Level 10
Gadolinium has outer configuration of [Xe]4f?5d¹6s².
Its third ionization energy is low due to highly exchange energy and hence stability of the half-filled f subshell.
New answer posted
4 months agoContributor-Level 10
MgH? is an ionic or saline hydride, GeH? is an electron precise hydride with 8 electrons around Ge, B? H? is an electron deficient hydride and HF is electron rich.
New answer posted
4 months agoContributor-Level 10
Molality = moles of solute / mass of solvent (kgs) ⇒ 1 = 0.5 / W (kgs)
Wsolvent (kgs) = 0.5 = 500 g
New answer posted
4 months agoContributor-Level 10
Carbon atoms in diamond are sp³ hybridized and those in graphite are sp² hybridized.
New answer posted
4 months agoContributor-Level 10
Li is used in making batteries, liquid Na is a coolant in nuclear reactors. Cs is used in photoelectric cells and KOH as a base is used to absorb acidic oxides like CO?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers