Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Density of metal a very specific and depends upon many factors.
Li -> 0.53 gm/cm3
Na -> 0.97 gm/cm3
K -> 0.86 gm/cm3
Rb ->1.53 gm/cm3
Cs -> 1.90 gm/cm3
New answer posted
3 months agoContributor-Level 10
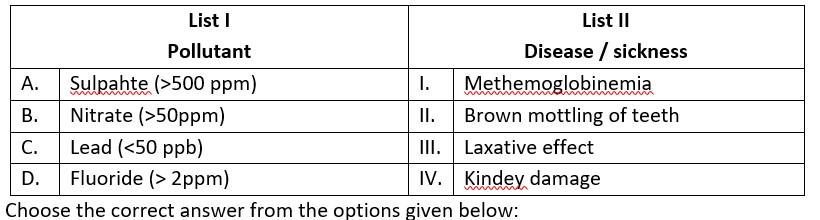
(A) Sulphate (>500 ppm) ® causes laxative effect that leads to dehydration.
(B) Nitrate (> 50 ppm) ® causes methemoglobinemia, skin appears blue.
(C) Lead (>50 ppb) ® It damage kidney and RBC.
(D) Fluoride (>2ppm) ® It causes brown mottling of teeth.
New answer posted
4 months agoContributor-Level 10
Be2Cl4 is Lewis acid and Al2Cl6 has complete octet. Be and Al are amphoteric metal therefore dissolve in acid as well as alkaline solution and form beryllate and aluminate ions in excess alkali.
New answer posted
4 months agoContributor-Level 10
Depending on the nature of reducing agent H2O2 can act as oxidizing agent in both acidic as well as basic medium.
Density of D2O = 1.1 gm/cc
Density of D2O2 = 1.45 gm/cc
New answer posted
4 months agoBeginner-Level 5
The reason why bond angle is larger in than are given below.
- In , there is one lone pair on the nitrogen atom increases repulsion, while the lone pair on phosphorus is in a higher energy orbital and causes less repulsion.
- Nitrogen is a small and electronegative atom whereas phosphorus is larger and less electronegative than nitrogen.
- In , the bonding orbitals are nearly pure p-orbitals, which are less directionals–p hybridization, on the other hand, the bond pairs remain fairly directed, leading to a larger bond angle.
Hence, the bond angle in is about 107° ( less than the ideal tetrahedral 109.5°) due to lone pair pres
New answer posted
4 months agoContributor-Level 10
(a) Polluted water has low value of dissolved oxygen, but high value of B.O.D., because chemical and organic matter uses dissolved oxygen to decompose.
(b) Eutrophication is result of excessive growth of weed in water bodies, which consumes dissolved oxygen, thus decreases oxygen.
New answer posted
4 months agoContributor-Level 10
Cu is least reactive metal and it lies below hydrogen in reactivity series.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers