Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Size of hydrated ion
Size of hydrated ions
Size of hydrated ions ;
Ionic mobility ;
New answer posted
4 months agoContributor-Level 10
To represent in detail NCERT ideas of organic chemistry basics (Class 11, Chapter Organic Chemistry - some basic principles and methodologies), you can use a variety of superb online resources. Sites such as Khan Academy provide entire notes, video recorded lectures and solved examples which deconstructs hard topics such as nomenclature, isomerism, reaction mechanisms and the effect of electron displacement. Physics Wallah and Aakash also have detailed revision videos as part of the NCERT syllabus which goes into details about these base concepts. Going through the platforms will give you deeper insights than the textbook.
New answer posted
4 months agoContributor-Level 10
The solution to the Class 11 chemistry chapter 9 exercise can be found on several education sites and that may be on 9 septuplets or 9 Octuplets (either hydrogen or hydrocarbons) depending upon the edition of NCERT syllabus you are following. Some of the best platforms by the likes of Physics Wallah, and Tiwari Academy have complete NCERT solutions that can be found in PDF format or even as a step by step instructions. Video solutions are also provided in the YouTube channels of CBSE Class 11 Chemistry.
New answer posted
4 months agoContributor-Level 10
2O3(g) -> 3O2
t = 0 a moles 0
-0.5a mole +0.75a mole
At Eq. 0.5a mole 0.75a mole
Total moles at eq = 0.5a + 0.75 a = 1.25a
New answer posted
4 months agoContributor-Level 10
Phosphorous can expand its covalency beyond 4, as it has vacant 3d orbitals. Nitrogen can't expand its covelency beyond 4, as it does not posses vacant 3d- orbitals.
New answer posted
4 months agoContributor-Level 10
Fire extinguisher contains sodium Bicarbonate (NaHCO3), also known as baking soda.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers

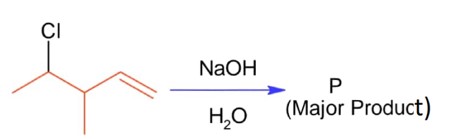
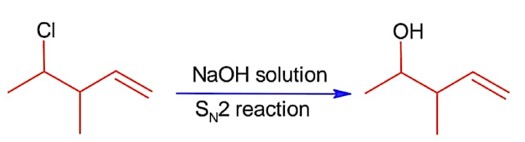 ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff
ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff