Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
Chapter 2 is Structure of Atom, aka the OG Science breakdown of how literally everything exists. You'll learn stuff like Bohr's model, orbitals, quantum numbers — all the nerdy magic that makes atoms behave. Sounds heavy, but once you get the hang of it, it lowkey feels like unlocking the Matrix. Trust the process, and maybe don't sleep through this one.
New answer posted
4 months agoContributor-Level 10
NCERT solutions for class 11 Chemistry chapter 9 hydrocarbon are crucial for understanding the fundamental principle of organic chemistry including the classification nomenclature and reaction of alkanes and alkyne and their vital role in daily life as fuse and in the manufacturing of polymer and other products
New answer posted
4 months agoContributor-Level 10
Ka for C3H7COOH = 2 * 10-5
=5 – 0.3 = 4.7
pH of 0.2 (M) solution =
Ans 27
New answer posted
4 months agoContributor-Level 6
Here are some of the best NCERT Class 11 Chemistry Chapter 11 exercise solutions you can download and study .
1. LearnCBSE
Provides detailed, step by step solutions covering every question—from Intext to Exercise problems.
Available in English, per the latest NCERT syllabus for 2023–24 & 2025- 2026
New answer posted
4 months agoContributor-Level 10
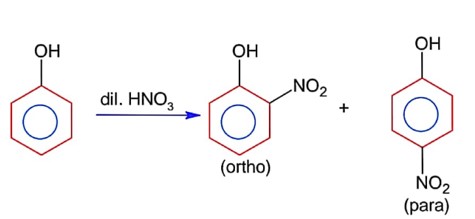
Ortho product has intramolecular H- bonding & para product has intermolecular H- bonding. Thus it can be separated by steam distillation due to difference in B.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers