Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Sodium reacts with Dihydrogen to form sodium hydride which is a crystalline ionic solid.
2Na + H2 → 2Na + H-
It reacts with H2O to produce H2 gas
2NaH+2H2O → 2NaOH+2H2
Although Na+H- does not conduct electricity in the solid state, the electrolysis of its melt produces H2 at the anode and Na at the cathode.
At cathode At anode
Na + H- (l) → &nbs
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: BF3 does not have a proton but still it acts as a Lewis acid as it is an electron pair acceptor and reacts with NH3by accepting its lone pair of electrons. The reaction can be represented by Coordinate bond is formed between BF3 and NH3. It can be represented as-
[ BF3 ← :NH3]
Nitrogen acts as lone pair donator in this combination
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Conductance of a solution depends upon the number of ions present in the solution since sodium chloride is an ionic compound when mixed in water it ionizes completely forming an aqueous electrolytic solution. Hence, the solution conducts electricity while sugar being a non-electrolyte does not ionize in water and when concentration of NaCl is increased, more Na+ and Cl- ions will be produced. Hence, conductance increases.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Conjugate acid and conjugate base can be shown as-
HCl(aq) + H2O (l) ? H3O+(aq) + Cl-(aq)
acid base conjugate conjugate
acid base
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
It is lithium hydride (LiH) because it has significant covalent character due to the smallest alkali metal, LiH is very stable. It is almost nonreactive towards oxygen and chlorine. It reacts with Al2Cl2 to form lithium aluminium hydride.
8LiH+ Al2Cl6 → 2LiAlH4 → LiCl
New answer posted
5 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (a) As we know,
ΔG= ?G? +RTlnQ
?G? = Change in free energy as the reaction proceeds,
ΔG = Standard free energy change,
Q = Reaction quotient,
R = Gas constant,
T = Absolute temperature.
Since, ?G? =−RTlnK
∴ΔG=−RTlnK + RTlnQ = RTlnKQ?
If Q
If Q=K, ΔG=0, reaction is in equilibrium and no net reaction is there.
(b) When we increase the pressure equilibrium will shift in forward direction it means Q
Types of Chemical Reactions :
There are 4 main types of chemical reactions. These are-
- Combination
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
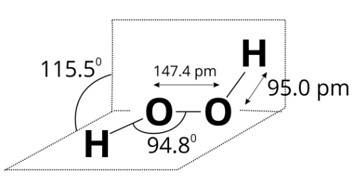
Since, a colourless liquid 'A' contains only hydrogen and oxygen and decomposes slowly on exposure to light but is stabilised by addition of urea, therefore, liquid A may be hydrogen peroxide. A is H2O2.
Structure of H2O2 is given below.
New answer posted
5 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The equation can be written as-
Ap+x + Bq- y ? xAp+ (aq) + yBq-
S moles of A and B dissolves to give x S moles of Ap+ and y S moles of Bq-.
Ksp = [Ap+]x [Bq-]y = [x5]x [y5]y
= xx y y 5 x+y
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Mass of H2O2 = 68 g.
Mol. Mass of H2O2=34gmol-1
∴1L of M solution of H2O2 will contain H2O2=34*5 g
∴2L of 5 M solution will contain H2O2=34*5*2=340g
or 200 mL of 5 solution will contain H2O2 = 200 = 34 g
Now 2H2O2 → 2H2O+O2
64 g 32g
Now 68 g of H2O2 on decomposition will give O2=32g
∴34g of H2O2 on decomposition will give O2 = 34 =16 g
New answer posted
5 months agoContributor-Level 10
(i) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols
2-ethylanthraquinol ↔ H2O2+ oxidised product
2Fe2+ (aq)+2H+ (aq)+H2O2 → Fe3 (aq)+2H2O (l)
PbS (s)+4H2O2 (l) → PbSO4 (s)+4H2O (l)
(ii) Reducing action of hydrogen peroxide
2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2
HOCl+H2O2 → H3O++Cl-+O2
Oxidising action of hydrogen peroxide
2Fe2++H2O2→ 2Fe3++2OH-
Mn2++H2O2 → Mn4++2OH-
Acidic properties of H2O2
I2+H2O2+2OH-→ 2I-+2H2O+H2
2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH-
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
