Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: According to Le Chatelier's principle, when we raise the temperature, it shifts the equilibrium to left and decreases the equilibrium concentration of ammonia since it is an exothermic reaction. In other words, low temperature and high pressure is favourable for high yield of ammonia. There will be no change in equilibria on addition of argon (Ar).
New answer posted
5 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The values of Kc and Qc are itself sufficient to explain the direction of reaction and less than or greater than one another decides the direction in which reaction will proceed as follows-
(i) As Qc < Kc, the reaction proceeds in the forward direction.
(ii) If Qc > Kc, the reaction will proceed in the direction of reactants (reverse reaction).
(iii) If Qc = Kc, no net reaction occurs.
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out.
Uses of H2O2:
(i) As an antiseptic it is sold in the market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per - carbonate. It is employed in the industries as a bleachin
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
D2O can be prepared by prolonged electrolysis of water. Due to high molecular mass D2O differs from water.
NaOH+D2O → NaOD+HOD
HCl+D2O → DCl+ HOD
NH4Cl+D2O → NH3DCl+HOD
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol-1 | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant | 78.39 | 78.06 |
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
New answer posted
5 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (iv)
Both the statements given in the assertion and reason are false.
The energy required to break the first O-H bond is not same than that of the second O-H bond in water molecule, that means the bond enthalpies of the two O-Hare different. This is because the electronic environment around the oxygen atom in water molecules is not the same even after the O-H bond breaks.
New answer posted
5 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (i)
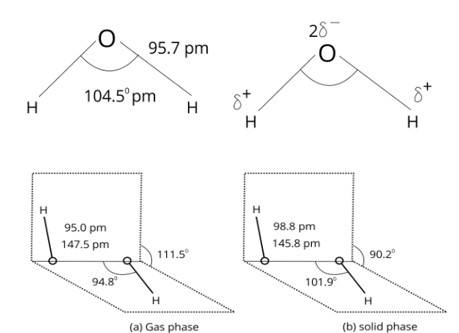
The statements given in the assertion and reason, both are correct and the reason given is the correct explanation for the assertion. In NH3 only one lone pair of electron is present and due to that lone pair-bond pair repulsion occurs which forms a distorted tetrahedral geometry (pyramidal) .
In H2O two lone pairs of electrons are present on the oxygen atom and due to this lone pairlone pair repulsion occurs which shifts the bonds and forms a bent geometry. The lone pair-lone pair repulsion is more than the lone pair-bond pair repulsion. Thus, bond angle i
New answer posted
5 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (i)
Both the statements given in assertion and reason are correct and the reason given is the correct explanation for the assertion. The sodium chloride formed by the action of chlorine gas on the sodium metal has a complete octet. The valence shells of both the atoms are fulfilled, that is why attains an octet.
New answer posted
5 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Tetrahedral (c) sp3
(ii) Trigonal (a) sp2
(iii) Linear (b)sp
Explanation :-
(i) A tetrahedral molecule has four electron pairs and these make sigma bonds with each other. The s and p orbitals overlap with each other thus forming a sp3 hybridized molecule.
(ii) There are two possibilities for the central atom to have sp2 Either all the bonds are in place i.e, the bond pairs are all sigma bonds or pi bonds or there are only two bonds and one lone pair of electrons.
(iii) The sp hybridization involves the mixing of the val
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
