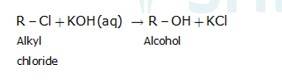Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
7.44
Nitrogen does not have d-orbital to expand its octet. So, it cannot have coordination number greater than 4. But, phosphorous has d-orbital and can extend its octet and form R3P = O. Therefore, R3P = O exist but R3N = O does not.
New answer posted
6 months agoContributor-Level 10
7.43
Among the group 15 elements, N has the highest electronegativity, because of which there is high electron density around N. This causes repulsion between the electron pairs around causing high HNH angle value.
As we go down the group, the electronegativity of the elements decease and the bond angle also decreases due to lesser repulsion.
New answer posted
6 months agoContributor-Level 10
7.42
To draw the resonating structure of NO2 No. of valence electrons for N = 5
No. of valence electrons for O = 2 * 6 = 12
Total no. of valence electrons = 5 + 12 = 17
The N and O atoms are arranged in such a way that the less electronegative atom N is placed as the central atom.
Electron pairs are placed between bonds and distributed around the atoms to form octet as shown. Both the O atoms have their complete octet but N has only 5 electrons.

A pair of electrons is transferred from O to the bond between O and N. Both the O atoms have their complete octets and N has 7 electrons.

The resonating structures of NO2 can be shown as:

New answer posted
6 months agoContributor-Level 10
7.41
Copper metal on reaction with HNO3 gets oxidized and give different by-products depending on the temperature, concentration of the acid and the copper metal undergoing oxidation.
The reaction of copper with concentrated and dilute HNO3 is as shown below:
3Cu + 8HNO3 (did.) 3Cu (NO3)2 + 2NO +4H2O
Cu + 4HNO3 (conc.) Cu (NO3)2 + 2NO2 +2H2O
New answer posted
6 months agoContributor-Level 10
This is a classic question from the chapter Haloalkanes and Haloarenes. Let us solve each one as follows:
(1) When n - butyl chloride is treated with alcoholic KOH, the formation of but - l - ene takes place. This reaction is a dehydrohalogenation reaction.
When N-butyl chloride or 1-chlorobutane is treated with alcoholic potassium hydroxide (KOH), elimination reaction takes place. This leads to alkene formation. 1-butene is the major product here.
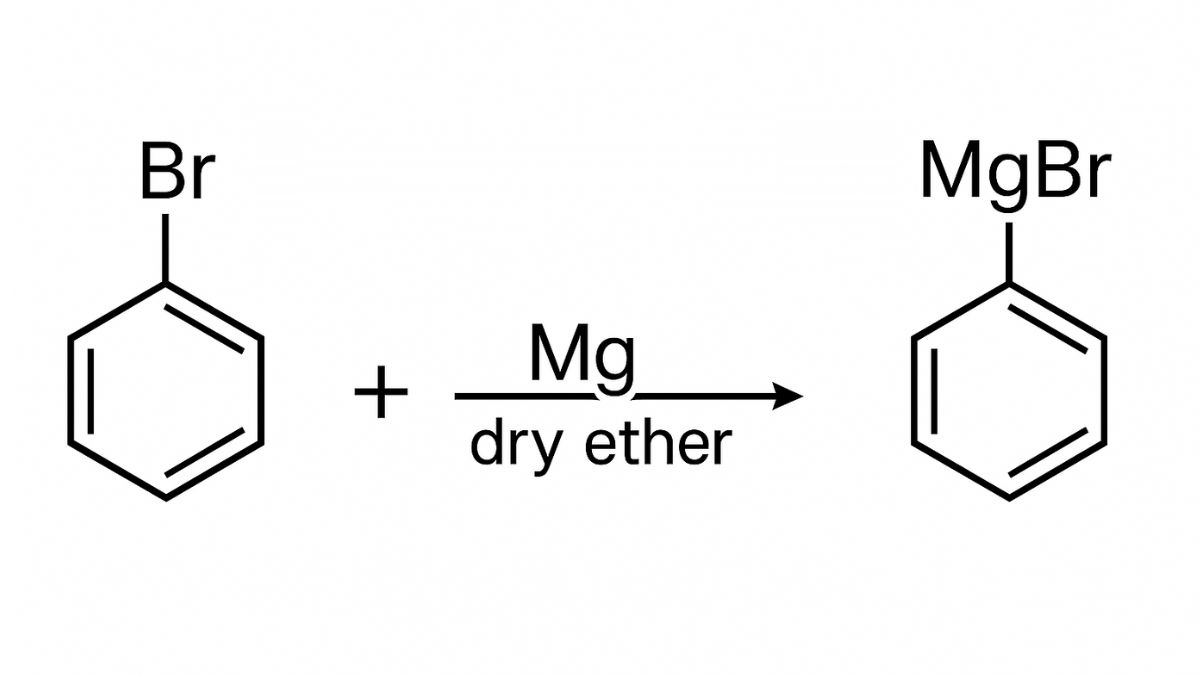
(ii) When Bromobenzene is treated with Mg in the presence of dry ether, it undergoes a reaction to produce phenylmagnesium bromide which is the Grignard reagent. This reagent is very re
New answer posted
6 months agoContributor-Level 10
7.40
Ammonia is manufactured industrially by Haber's process. Nitrogen from gas is combined with hydrogen derived from natural gas (methane) in the ratio 1:3 giving rise to ammonia. The reaction is reversible and exothermic.

The optimum conditions for the manufacturing of ammonia are pressure 200*105 Pa, the temperature of 4700 K and iron oxide catalyst with a small amount of Al2O3 and K2O.
New answer posted
6 months agoContributor-Level 10
7.39
Nitrogen is prepared in the laboratory by heating aqueous ammonium chloride (NH4Cl) with sodium nitrite (NaNO2) to form ammonium nitrite (NH4NO2), which is unstable. Ammonium nitrite breaks down to form nitrogen and water.
NH4Cl (aq) + NaNO2 (aq) - NH4NO2 + NaCl (aq)
NH4NO2 - N2 (g)+ H2O (l)
Small amounts of NO and HNO3 are also produced which can be removed by passing nitrogen gas through aqueous sulphuric acid containing potassium dichromate.
New answer posted
6 months agoContributor-Level 10
There are two primary alkyl halides having the formulaC4H9Br, They are n - butyl bromide and isobutyl bromide.
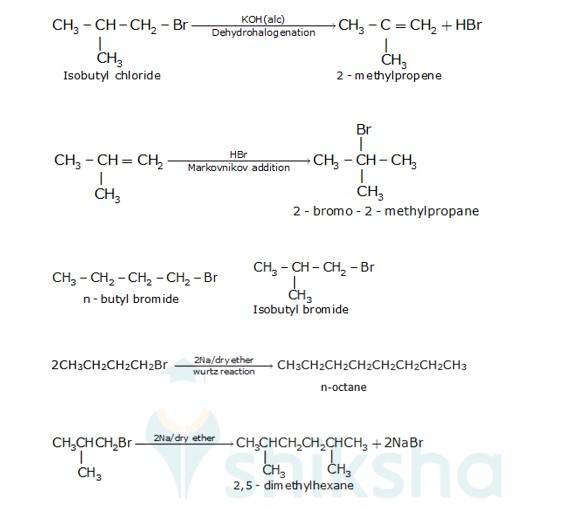
Therefore, compound (a) is either n-butyl bromide or isobutyl bromide.
Now, compound (a) reacts with Na metal to give compound (b) of molecular formula, C18H18 which is different from the compound formed when n-butyl bromide reacts with Na metal.
Hence, compound (a) must be isobutyl bromide. Thus, compound (d) is 2, 5-dimethylhexane.
It is given that compound (a) reacts with alcoholic KOH to give compound (b). Hence, compound (b) is 2- methylpropene.
Also, compound (b) reacts with HBr to give compound (c) which is an isomer of (a)
New answer posted
6 months agoContributor-Level 10
An aqueous solution, KOH almost completely ionizes to give OH-ions. OH- ion is a strong nucleophile (see the imp. note below), which leads the alkyl chloride to undergo a nucleophilic substitution reaction to form alcohol.
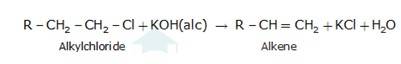
On the other hand, an alcoholic solution of KOH contains alkoxide (RO-) ion, it is a strong base. Thus, it can abstract a hydrogen ion from the beta-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.OH- ion is a much weaker base than RO- ion. Also, OH- ion is highly solvated (because more energy is released on solvation)in an aqueous solution and as a result, the basic character of OH-io
New answer posted
6 months agoContributor-Level 10
7.38
The electronegativity of N (3.0) is higher than that of P (2.1). Due to this N-H bond is more polar than a
P-H bond. Also, P and H have the same electronegativity of 2.1 i.e., the P-H bond is non-polar. Therefore, PH3 does not form hydrogen form.
The structure of NH3 and PH3 with their electronegativity is represented below.

Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers