Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
The order of increasing boiling point is
- Chloromethane (CH3Cl)< Bromomethane (CH3Br) < Dibromomethane (CH2Br2)< Bromoform (CHBr3)
- Isopropyl chloride (C3H7Cl)< 1-Chloropropane (C3H7Cl) < 1-Chlorobutane (C4H9Cl)
Generally, boiling point increases with the molecular weight of the compound and decreases with the branching of the chain
New answer posted
6 months agoContributor-Level 10
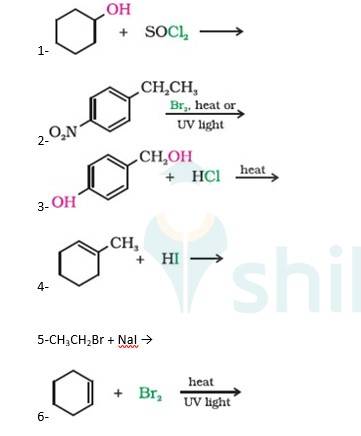
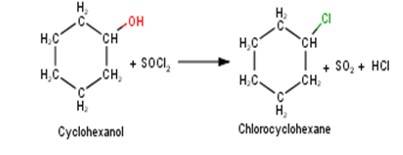
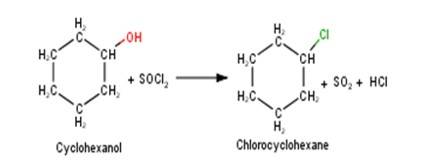
A 10.5 1. Cyclohexanol will react with thionyl chloride to form Chlorocyclohexane with the evolution of sulphur dioxide and hydrogen chloride gas as shown below :
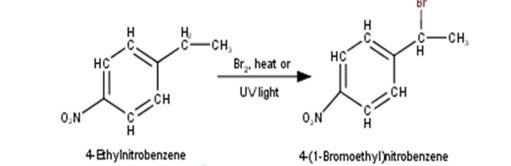
2. 4-Ethylnitrobenzene will undergo benzylic bromination in presence of heat or light. Now, as benzylic radicals are more stable therefore benzylic hydrogen is abstracted. Hence, the reaction yields 4-(1- Bromoethyl)nitrobenzene as a product
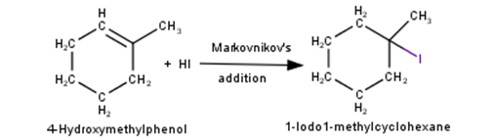
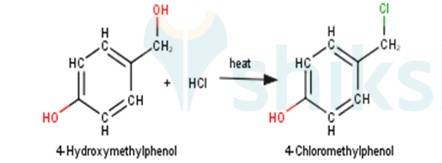
3. 4-Hydroxymethylphenol will react with hydrochloric acid under thermal conditions to yield 4- Chloromethylphenol a shown in the reaction below:
4. 1-Methylcyclohexene will react with hydrogen iodide via markovnikov's addition mechanism to
New answer posted
6 months agoContributor-Level 10
To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because of the fact that replacement of any H-atom leads to the formation of the same product. Therefore the isomer is 2,2-dimethylpropane.
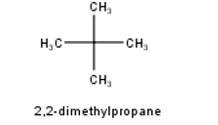
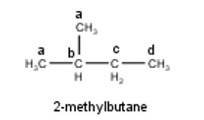
To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms. Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane as shown below : -
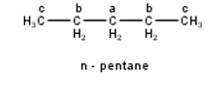
To have four isomeric monochlorides, the isomer of the alkane of the molecu
New answer posted
6 months agoContributor-Level 10
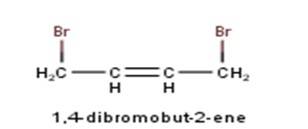
10.3 There are four different dihalogen derivatives of propane. The structures of these derivatives are as shown below: -
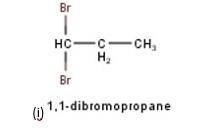

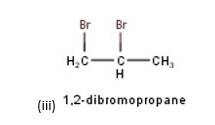
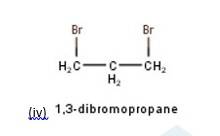
New answer posted
6 months agoContributor-Level 10
In the presence of sulphuric acid (H2SO4), KI produces HI as follows : -
2KI + H2SO4 → 2KHSO4 + 2KI
Since H2SO4 is an oxidising agent, it oxidises HI (produced in the reaction to I2)
2HI + H2SO4 → I2 + SO2 +;
As a result, the reaction between an alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidising acid such as H3PO4 is used in the reaction to get the desired product.
A few things you can remember here, while solving NCERT Solutions for Haloalkanes And Haloarenes.
Sulphuric acid is a powerful oxidising agent. We know this because the su
New answer posted
6 months agoContributor-Level 10
7.31
In interhalogen compound ICl and I2 the atoms are bonded by covalent bonds
Reactivity refers to the rate at which a chemical species will undergo reaction in time. For reaction to take place the compound needs to be broken into separate elements first, then the individual elements react with other elements to form new compounds. So any compound which can easily break into their individual elements can react faster.
The covalent bonds between dissimilar atoms I and Cl atoms in ICl are weaker than between similar atoms in I2. Therefore the bond between ICL will break easily so the I and Cl atom will be easily available to form another
New answer posted
6 months agoContributor-Level 10
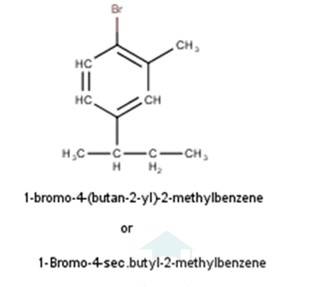
10.1 From the name of the compound it is clear that the parent ring is pentane and chloro and methyl groups are attached in the straight chain at 2nd and 5th position respectively. Hence, the structure is as follows:
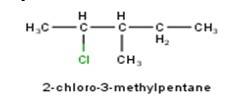
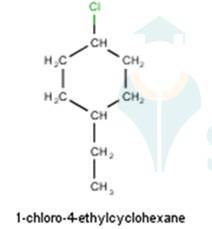
From the name of the compound given it is clear that the parent group is hexane with 2 attachments namely chloro and ethyl groups at 1 and 4 positions respectively. Hence, the structure is as follows: -
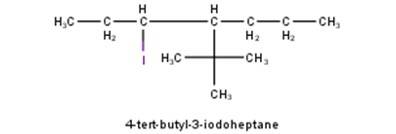
From the name of the compound given it is clear that the parent group is heptane with tertiary butyl and iodine groups attached at 4 and 3 positions respectively. Hence, the structure is as follows: -
From the name of the comp
New answer posted
6 months agoContributor-Level 10
8.48 To calculate magnetic moment of the complex species, we use the spin formula:
μ =√n(n+2) BM
When n= 1 | ⇒ μ = √1(1+2) ⇒ u= √3⇒ u=1.73 BM |
When n=2 | ⇒ μ = √2(2+2) ⇒ μ = √8 ⇒ μ = 2.83 BM |
When n= 3 | ⇒ μ = √3(3+2) ⇒ μ = 15 ⇒ μ = 3.87 BM |
When n = 4 | ⇒ μ = √ 4(4+2) ⇒ μ = √24 ⇒ μ = 4.899 BM |
When n= 5 | ⇒ μ = √5(5+2) ⇒ μ = √35 ⇒ μ = 5.92 BM |
1. [K4 [Mn(CN)6]
⇒μ = 2.2 BM (given)

We can see from the above calculation that the given value(2.2) is close to n=1. It means that it has only one unpaired electron Also in this complex Mn is in +2 oxidation state,i.e., as Mn2+.Thus when CN- ligands approach Mn2+ ion, The electrons in 3d do not pair up.
The atomic number of Manganese (Mn) is Z = 25
The electronic configuration of 25Mn= [Ar] 3d5 4s2
And, the electronic configuration of Mn2+=[Ar] 3d5
Thus CN- is a strong ligand.
The hybridization involved is d2sp3 forming inner orbital oc
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
8.47 The given statement is true as explained below:
1. Atomic radii of the heavier transition elements (4d and 5d series) are larger than those of the corresponding elements of the first transition series through those of 4d and 5d series are very close to each (Lanthanoid contraction)
2. Due to stronger intermetallic bonding (M-M bonding), the melting and boiling points of heavier transition elements are greater than those of the first transition series
3. The ionization enthalpies of 5d series are higher than the corresponding elements of 3d and 4d
4. The heavier transition elements form low spin complexes whereas the elements of t
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers