Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
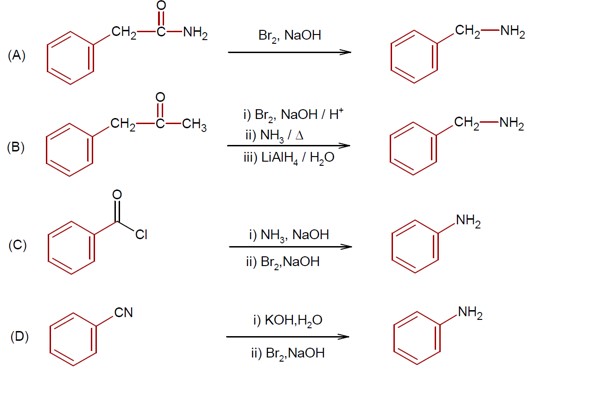
R-CONH? + Br? + 4NaOH → R-NH? + 2NaBr + Na? CO? + 2H? O
This reaction is the Hoffmann bromamide degradation, in which an amide is converted to a 1° amine.
New answer posted
3 months agoContributor-Level 9
The E° value for Ce? /Ce³? is +1.74 V, which suggests that Ce? is a strong oxidant, reverting to its common +3 oxidation state. So, Ce³? is more stable than Ce?
New answer posted
3 months agoContributor-Level 9
The size of the Bk³? ion is less than the Np³? ion because Berkelium (Bk) lies beyond Neptunium (Np) in the actinoid series, and the size variation here is because of the actinoid contraction.
New answer posted
3 months agoContributor-Level 9
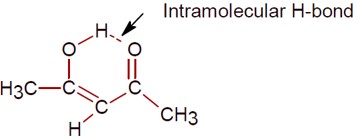
The enol form of acetone exists in less than 0.1% quantity, since its keto form is highly stable. But in the case of acetylacetone, the enol form is stabilized by intramolecular H-bonding, so its quantity increases to approximately 15%.
The intramolecular H-bond in the enol form of acetylacetone is shown.
New answer posted
3 months agoContributor-Level 9
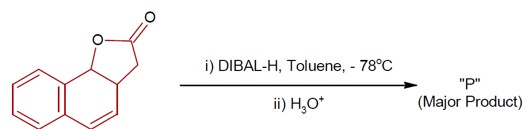
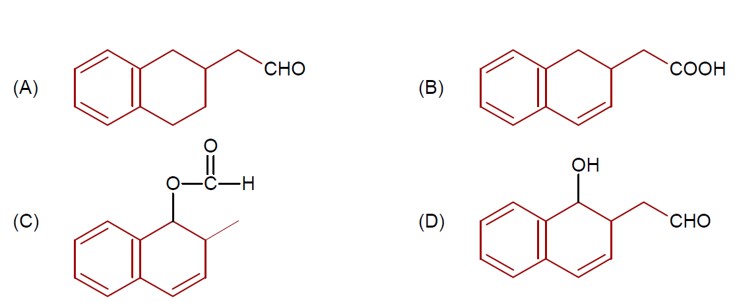
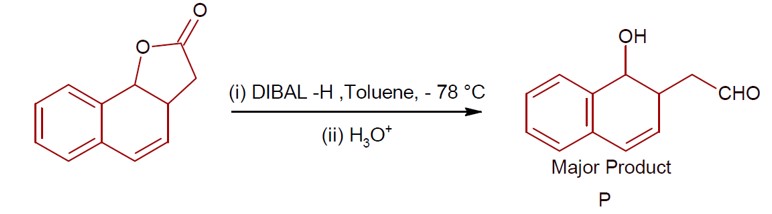
DIBAL-H at low temperature in a non-polar solvent, followed by hydrolysis, reduces esters to an aldehyde and an alcohol as a byproduct. The reaction shown is:
New answer posted
3 months agoContributor-Level 10
First, the number of unit cells in the given mass is determined. In an HCP structure, there are 6 atoms and 18 total voids (6 octahedral + 12 tetrahedral) per unit cell. Multiplying the number of unit cells by 18 gives the total number of voids. The result is14.9 x 10²¹, which is rounded.
New answer posted
3 months agoContributor-Level 10
Urea-formaldehyde resin is used for wood laminates because of its durable and unbreakable properties.
New answer posted
3 months agoContributor-Level 9
Solubility of CdSO? is water ; S = 8 * 10? M
Using Ksp = S²
Ksp = 64 * 10? M
Now,
CdSO? Cd²? + SO? ²?
in H? SO? (0.01M)
Ksp = [Cd²? ] [SO? ²? ]
64 * 10? = S? (S? + 0.01)
S? << 0.01
So, S? = 64 * 10? M.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers