P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Bartlett noticed that Xenon's first ionisation potential is nearly identical to that of oxygen and used Born-Haber calculations to predict the presence of a stable compound: Xe+ Pt F6-
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
MnO2 ( Black )+4HCl→MnCl2+2H2O+Cl2
Greenish yellow color
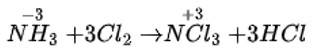
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
C+2H2SO4→CO2+2SO2+2H2O
Conc. H2SO4 oxidises C to produce two gaseous products.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
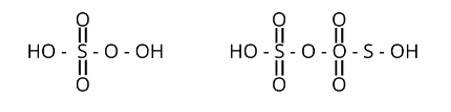
Peroxy linkage refers to the presence of a bond between oxygen and oxygen (O-O) in a molecule.
Only H2S2O6 and H2S2O7 of the sulphur oxoacids above have a peroxy linkage.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The C-atom in the CO32- ion undergoes sp2 hybridization. BF4, NH4+ and SO42- have a tetrahedral structure, whereas it has a triangular planar structure.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Na (H2)PO2
1+ (2x+1)+ x +2 (−2)=0
x−1=0
x=1
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The catalytic oxidation of ammonia produces NO gas, which is used to make HNO3. 4 moles of NH3 created 4 moles of NO in the equation below. As a result, the moles of NO produced by oxidising two moles of NH3 will be two moles.
4NH3 + 5O2→4NO + 6H2O.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
We get N2 in both situations when we heat ammonium dichromate and barium azide separately.
(NH4)2Cr2O7→Cr2O3 + 4H2O + N2
Ba (N3)2→Ba + 3N2.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
Bismuth is the sole element that has an inert pair effect. Only generates trihalides and has a +3 oxidation state. Florine, on the other hand, is small and has a high electronegativity.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The 'Brown Ring Test' is an identification and conformation test of the nitrate ion (NO3- ) using conc. H2SO4 and newly produced FeSO4. Place the NO3- containing sample in a test tube. Then, with gentle shaking, add conc H2SO4 dropwise, heat it briefly on a Bunsen burner, and add freshly made greenish FeSO4 Then, due to the creation of brown coloured [Fe (H2O)5 (NO)]SO4 complex, a brown ring forms in that test tube.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
