Physics
Get insights from 5.6k questions on Physics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation-
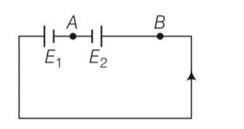
total resistance = 2+8= 10 ohm
I= = 0.2A
The direction of flow of current is always from high potential to low potential
If VB VA
VB-4V-0.2 8 = VA
VB-VA= 3.6V
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
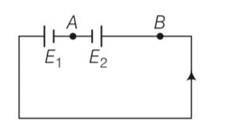
Explanation – in series combination of resistors current I =e/R+r
10I=
10 = ( )n n=10
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
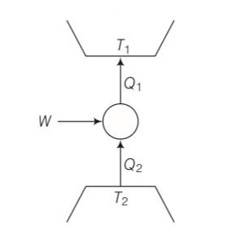
(a), (c) Q1= W+Q2
W=Q1-Q2>0
Q1>Q2>0
We can also write Q21
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
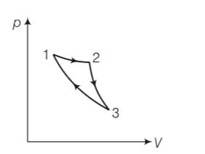
(a) the given process is a cyclic process i.e returns to the original state 1
Hence change in internal energy dU =0
dQ= dU+dW=0+dW
hence total heat supplied is converted to work done by the gas which is not possible by second law of thermodynamics.
(c) When the gas expands adiabatically from 2 to 3 . it is not possible to return to the same state without being heat supplied hence 3 to 1 cannot be adiabatic.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
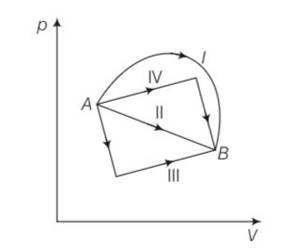
(b), (c) Change in internal energy for process A to B
dU=nCvdT=nCv (dT)=nCv (TB-TA)
work done from A to B = area under the PV curve which is maximum for path I
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a), (d) For isothermal dT= 0 so T=constant
For an ideal gas dU = change in internal energy = nCvdT=0
From first law of thermodynamics dQ= dU+dW
dQ= dW
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a), (b), (d) When the rod is hammered the external work is done on the rod which increases its temperature.
Heat is transferred to the gas in the small container by big reservoir at temperature T2
As the weight is added to the cylinder arrangement in the form of external pressure so it cannot reversed.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
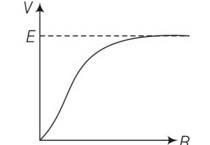
Explanation- according to the relation V=e-Ir and I=e/r+R
The graphical relation between voltage and resistance.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) Let us assume that T1, T2T3
According to questions there is no loss of heat in the surroundings
Heat lost by M3 = heat gained by M1+ heat gained by M2
M3s (T3-T)= M1s (T-T1)+M2s (T-T2)
T [M1+M2+M3]= M3T3+M1T1+M2T2
T =
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
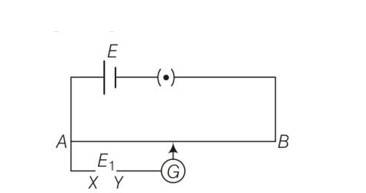
Explanation- If the current in auxiliary circuit (lower circuit containing primary cell) decreases, and potential difference across A and jockey/increases. Then deflection in galvanometer is one sided and the deflection decreased, while moving from one end 'A ' of the wire to the end 'S'.
And clearly this is possible only when positive terminal of the cell E1 is connected at X and E1>E.
(ii) If the current in auxiliary circuit increases, and potential difference across A and jockey J increases. Then also deflection in galvanometer shows one sided deflection.
And th
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers