Physics Thermodynamics
Get insights from 156 questions on Physics Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
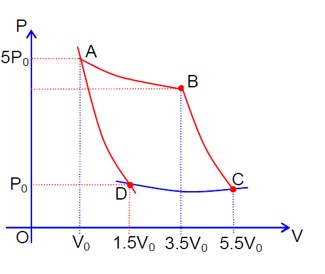
A→B & D→C (isothermal process)
So, TA = TB & TD = TC. Now B→C & D→A (adiabatic process)
|WBC| = nR/ (γ-1) (TB - TC)
|WAD| = nR/ (γ-1) (TA - TD) = nR/ (γ-1) (TB - TC)
∴ |WBC| = |WAD|
New answer posted
6 months agoContributor-Level 9
C? = dQ/ndT = (dU + pdV)/ndT
= C? + (pdV/ndT)
C? - C? = pdV/ndT = R - For ideal Gas [Box: PV = nRT, pdV = nRdT]
C? - C? = 1.1R - For Non – Idea gas (for Real gas)
And Real gas behaves as ideal gas at high temperature & low pressure.
∴ T_B > T_A
New answer posted
6 months agoContributor-Level 9
For adiabatic process
T? V? ¹ = T? V? ¹
⇒ T? (Al? )? ¹ = T? (Al? )? ¹
T? /T? = (l? /l? )? ¹ = (l? /l? )? /³? ¹ = (l? /l? )?
New answer posted
6 months agoContributor-Level 10
η = 1 - T? /T?
1/6 = 1 - T? /T? (i)
2η = 1/3 = 1 - (T? -62)/T? (ii)
By (i) and (ii)
(T? /T? ) = 5/6
1/3 = 1 - (T? /T? ) + 62/T? = 1 - 5/6 + 62/T?
1/3 = 1/6 + 62/T?
1/6 = 62/T?
T? = 62 * 6 = 372K = 99°C
New answer posted
6 months agoContributor-Level 10
In isothermal process, temperature is constant.
In isochoric process, volume is constant.
In adiabatic process, there is no exchange of heat.
In isobaric process, pressure is constant
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers