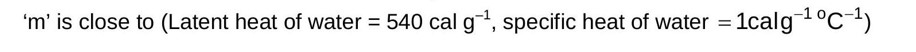Physics Thermodynamics
Get insights from 156 questions on Physics Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
After burning, heat exchange occurs between helium and atmospheric. Hence, irreversible, isothermal process.
New answer posted
6 months agoContributor-Level 10
The internal energy of one mole of an ideal gas is given by U = (f/2)RT, where f is the number of degrees of freedom.
For a non-linear triatomic molecule (assumed to be a rigid rotator), there are:
3 translational degrees of freedom.
3 rotational degrees of freedom.
Vibrational modes are generally not considered at temperature T unless specified.
Total degrees of freedom f = 3 + 3 = 6.
U = (6/2)RT = 3RT.
New question posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers