Physics Thermodynamics
Get insights from 156 questions on Physics Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoNew answer posted
7 months agoContributor-Level 10
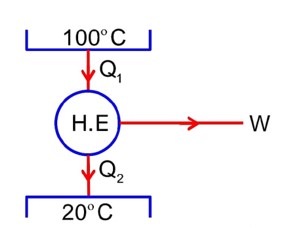
0.25 = 1 -
0.25 = 1 -
T1 = 400 k
Now, efficiency increases by 100%
= 0.25 * 2
= 0.50
0.50 = 1 -
(600 – 400) = 200 k or 200° C
New answer posted
7 months agoContributor-Level 10
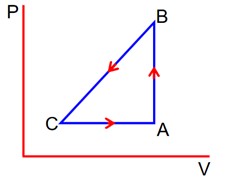
Given
QAB = +40J
QBC = 0J
QCA = -60J
WCA =?
WBC = -50J (on the gas)
UA = 1560J
1st Law on path A to B
WAB +
Þ 0 +
Þ UB = UA + 40
= 1560 + 40
UB = 1600 J
1st Low on path B
New answer posted
7 months agoContributor-Level 10
One of the most important Mechanical Engineering subjects is Thermodynamics, which deals with the concepts of heat energy and temperature, along with its conversion between different forms. You would come across a variety of books that will give you a basic understanding of the concept, but here are a few book suggestions for Mechanical Engineering freshers to grasp the basics of thermodynamics quickly
- Basic and Applied Thermodynamics by P. K. Nag
- Engineering Thermodynamics by S. K. Som & G. Biswas
- The Laws of Thermodynamics: A Very Short Introduction by Peter Atkins
New answer posted
7 months agoContributor-Level 6
In GATE XE (Engineering Sciences), Thermodynamics is one of the optional sections (XE-E), but admissions for M.Tech are usually branch-specific (like Mechanical, Thermal, Energy Engineering).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers