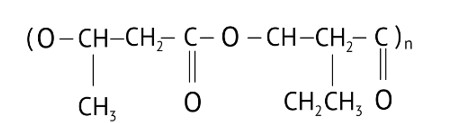Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
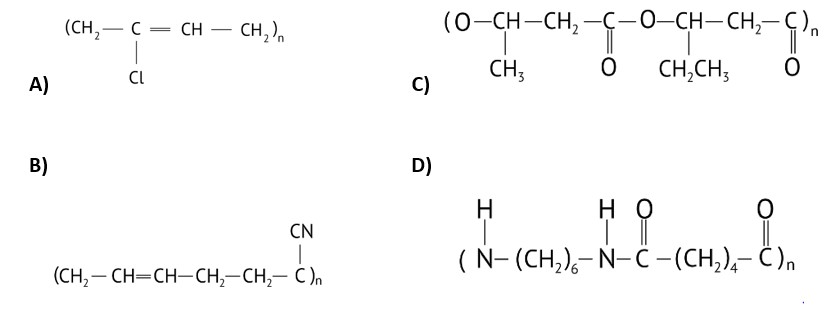
Option (B) and (C)
Explanation: Buna-S and neoprene are the polymers that needs at least one diene monomeric unit. Buna- S is a polymer formed from the polymerization of 1,3-butadiene and styrene. While, Neoprene is formed by the polymerisation of chloroprene.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
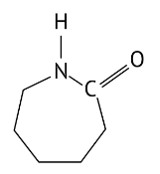
Option (D)
Explanation: The given structure is caprolactam. Nylon-6 is prepared from the monomer caprolactam. It is obtained by heating caprolactam with water at a high temperature.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (B)
Explanation: Low-density polythene is obtained from the polymerization of ethane under high pressure and temperature. Low-density polyethylene is tough, a poor conductor of electricity and has a highly branched structure.
Low-density polythene is not hard because branched structure prevents stacking and thus, reduces the intermolecular force of attraction.
So, option (B) is correct.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
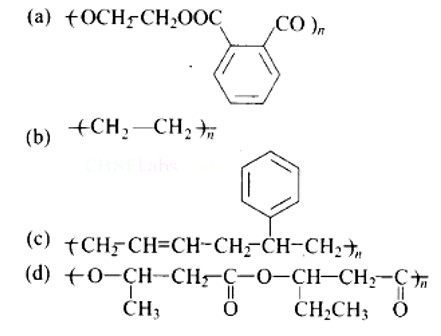
Option (A)
Explanation: Dacron or Terylene is formed by the condensation polymerization of ethylene glycol and phthalic acid with the elimination of water molecules. The structure of Terylene is,
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (C)
Explanation: PHBV (Poly beta-hydroxybutyrate co-beta-hydroxy valerate) is a biodegradable polymer. It undergoes bacterial degradation in the environment. The structure is shown as,
New answer posted
6 months agoContributor-Level 10
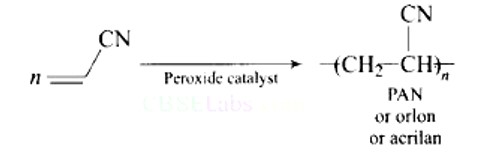
This is a multiple choice answer as classified in NCERT Exemplar
Option (B)
Explanation: Polyacrylonitrile is used as a wool substitute in the manufacture of commercial fibers such as orlon or acrilan.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A)
Explanation: Semi-synthetic polymers are obtained from natural polymers. Cellulose derivatives like cellulose nitrate, cellulose acetate rubber are examples of semi-synthetic polymers.
Cis-polyisoprene is not a semi-synthetic polymer while other examples are semi-synthetic polymer.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (D)
Explanation: Glycogen is a branched polymer of glucose found in the liver, muscles, and brain of animals.
New answer posted
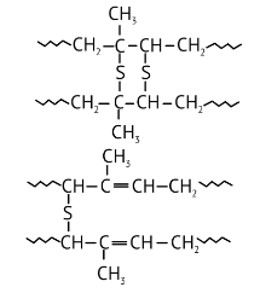
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: While manufacturing tyre rubber, 5 % sulfur is used as a crosslinking agent. A natural linear polymer of 2-methyl-1, 3-butadiene becomes hard on treatment with sulfur and forms vulcanised rubber. The structure of the product formed is,
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Thermoplastic polymer
Thermoplastic polymers are linear or slight branched-chain polymers. In this type of polymer, the strong intermolecular force of attraction is present between elastomers and fibers. This type of polymer softens on heating and hardens on cooling. For example, polythene, polystyrene and polyvinyl.
Thermosetting polymer
Thermosetting polymers are cross-linked polymers. These polymers on heating undergo cross-linking in molds. They become infusible on heating so, cannot be reused again. For example, Bakelite and urea-formaldehyde resins.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers