Polymers
Get insights from 154 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
10 months agoContributor-Level 10
15.11
It is polyvinylchloride. It is a homopolymer as it is made up of the same type of repeating monomeric units.
New answer posted
10 months agoContributor-Level 10
15.10
In chemical reactions when monomer units form chains or three–dimensional networks, it is known as polymerization. Various methods of polymerization are Step-growth polymerization, Chain-growth polymerization and Condensation polymerization.
New answer posted
10 months agoContributor-Level 10
15.9
The functionality of a monomer is defined as the number of bonds that a monomer's repeating unit forms in a polymer with other monomers. A linear polymer is formed by polymerizing if the functionality of monomer is two and is bifunctional (a thermoplastic).
New answer posted
10 months agoContributor-Level 10
15.8 Copolymers are those polymers that consist of more than one monomeric repeating unit. Some of the copolymers are Saran, Butadiene, Nitrile Rubber, Butyl Rubber, Viton and many more.
Homopolymers are those in which there is only one monomeric unit. Some of the examples of homopolymers are polypropylene, Polythene and many more.
New answer posted
10 months agoContributor-Level 10
15.7 Based on source, polymers can be classified as below:
Natural polymers: Those polymers obtained from plants and animals such as proteins, cellulose, starch and other resins.
Semi Natural polymers: Those polymers that are derivatives of natural polymers fall under this category. Cellulose Nitrate used as a propellant and in guncotton and Cellulose Acetate used in photography are prepared from Cellulose.
Synthetic polymers: Those polymers which are prepared in the laboratory are called synthetic Bakelite, Polythene, synthetic fibers and rubbers are some of its examples.
New answer posted
10 months agoContributor-Level 10
15.1
The word polymer comes from poly- (many) and -mer (part). Polymers are generally high molecular mass substances that have repeating units of smaller molecules. They may be a natural or synthetic macromolecule. The single molecular units of which a large chains are made is known as 'monomer'. These monomers generally have high molecular mass (103- 107u). Some examples of polymers are polythene, Bakelite, rubber, Buna-N and many more.
New answer posted
10 months agoContributor-Level 10
15.5
Different types of polymers have different intermolecular forces of attraction. Elastomers or rubbers have the weakest while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate intermolecular forces of attraction. Hence, the increasing order of the intermolecular forces of the given polymers is as follows:
Buna? S < polythene < Nylon 6, 6
Neoprene < polyvinyl chloride < Nylon 6
New answer posted
10 months agoContributor-Level 10
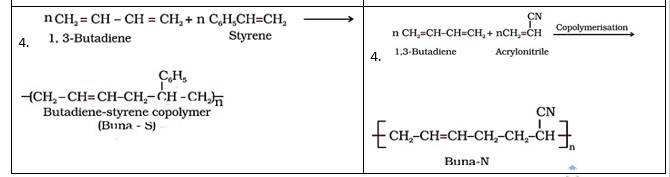
15.4
Buna-S | Buna-N |
1. It is formed from 1,3- butadiene and styrene in presence of sodium. | It is formed from 1,3-butadiene and acrylonitrile in presence of sodium. |
2. Bu refers Butadiene, Na refers Sodium and S refers Styrene | Bu refers Butadiene, na refers Sodium and N refers acrylonitrile |
3. It is used for making automobile tyres, rubber belts, etc. | It is used for manufacturing of tank linings, protective gloves etc. |
New answer posted
10 months agoContributor-Level 10
15.3
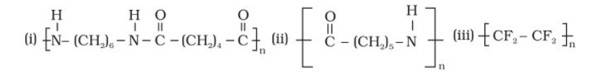
The monomer is OC- (CH2)5-NH known as Caprolactam. The cyclic structure of the monomer changes to linear to form the polymer, as shown below:
The monomer is Tetrafluroethene (CF2= CF2), the double bond breaks to form the polymeric
New answer posted
10 months agoContributor-Level 10
15.2
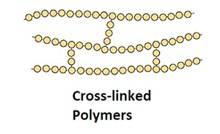
Polymers are classified based on structure, into 3 types:
Linear Polymer: They have a long and straight chain of Ex: high-density Polythene, Polyvinyl chloride

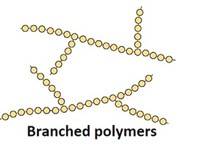
Branched-chain Polymers: They have linear molecular chains along with some Ex: less density polythene.
Cross-linked or network Polymers: In these polymers, strong covalent bonds are between the linear chains. Generally, they contain 2 or 3 types of functional groups. Ex: Bakelite, melamine.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers
