Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:

New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
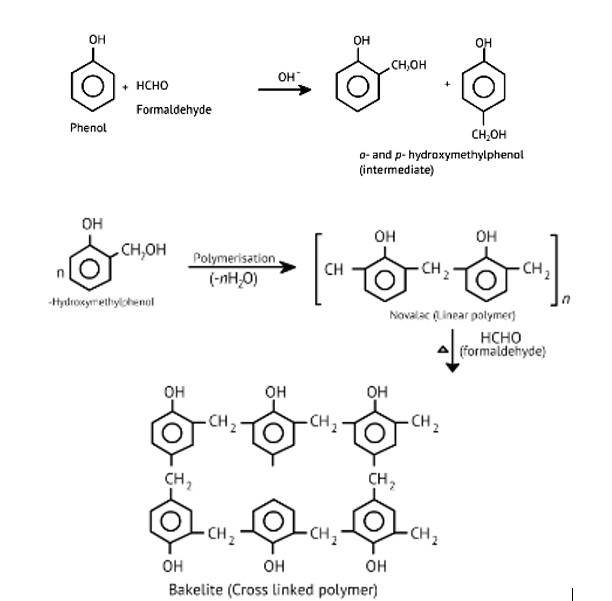
The oldest synthetic polymers are phenol-formaldehyde polymers. These are made by combining phenol and formaldehyde in the presence of either an acid or a base catalyst. The reaction begins with the formation of o-and/or p hydroxymethyl phenol derivatives, which then react with phenol to form compounds with shaving rings connected by –CH2 groups. The first product could be a linear product, such as Novolac.
When heated with formaldehyde, Novolac crosslinks to form Bakelite, an infusible solid mass.
It is used to make combs, phonograph records, electrical switches, and
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
In rubber, the polymer chain are held together by the weakest force of attraction. While, in plastic, the strong intermolecular force of attraction is present between elastomers and fibres.
In rubber, a few crosslinks are introduced between the chain, while plastics are linear or slightly branched molecules which gets soft on heating and hard on cooling.
New question posted
6 months agoNew question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
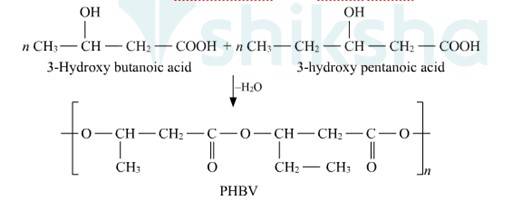
1.Poly-hydroxybutyrate-co-β-hydroxyvalerate (PHBV)
It's made by copolymerization 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV is used in specialty packaging, orthopaedic devices, and controlled drug release. In the environment, PHBV is degraded by bacteria.
Nylon 2-Nylon 6: It is a biodegradable alternating polyamide copolymer of glycine and amino caproic acid.
Biopolymers are natural polymers found in plants and animals such as protein, fat, cellulose, and so on.
Biodegradable polymers are polymers that contain functional groups that are similar to those foun
New question posted
6 months agoNew answer posted
7 months agoContributor-Level 10
15.24
Biodegradable polymers are designed to degrade into simpler components like water, CO2, Nitrogen etc. upon disposal by the action of living organisms. Extraordinary progress has been made in the development of practical processes and products from polymers such as starch, cellulose, and lactic acid. The need to create alternative biodegradable water- soluble polymers for down-the-drain products such as detergents and cosmetics has taken on increasing importance. Aliphatic polyesters are one of the important classes of biodegradable polymer. Example. Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV)
New answer posted
7 months agoContributor-Level 10
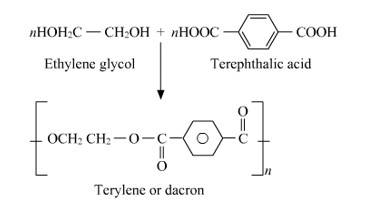
15.23
Dacron is a polyester batting that should be added to any foam surface so that it will not be exposed directly to the fabric. Dacron has many indispensable qualities like batting reduces the friction foam has, and thus reduces wear to fabric and because polyester batting remains springy, it is ever ready to put some light pressure against fabric. This means that even as the fabric stretches with age (and always happens) batting will push against the fabric and keep wear-worn waves from developing.
New question posted
7 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers