Solutions
Get insights from 202 questions on Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Let volume of solution = x ml
So mass of solution = 1.2x
And mass of water = x gm
Mass of solute = 0.2x
Molality = (W_solute * 1000) / (M_solute * W_solvent) = (0.2x * 1000) / (40 * x) = 5 m
New answer posted
5 months agoContributor-Level 10
? = CRT
2.42 * 10? ³ = ( (1.46/M_polymer) / 0.1 ) * 0.083 * 300
M_polymer = (1.46 * 0.083 * 300) / (2.42 * 10? ³ * 0.1)
= 14.96 * 10? gm = 15 * 10? gm
New answer posted
5 months agoContributor-Level 10
ΔTb = 0.6 K
ΔTb = Kb * m = Kb * (wt. * 1000)/ (mol wt. * Wsolvent (gm)
0.6 = 5 * (3 * 1000)/ (mol wt. * 100)
∴ mol wt. = (15 * 10)/ (0.6) = 1500/6
= 250 g/mole
New answer posted
6 months agoContributor-Level 10
Depression in freezing point will be maximum for Al2 (SO4)3 since its Van't Hoff factor is the highest. So its solution will have the lowest freezing point.
New answer posted
6 months agoContributor-Level 10
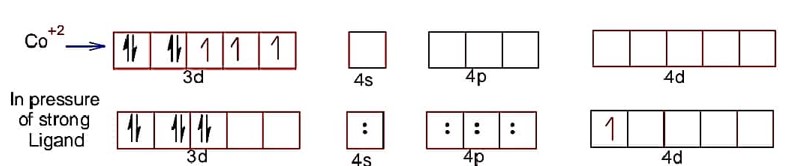
Hence, electronic configuration of CO2+ is
spin magnetic moment =
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
KCl solution has molality (m) = 3.3, [Means 3.3 mol of KCl dissolved in 1 kg of solved]
Total mass of solution = mass of solute + mass of solvent
= 3.3 * 74.5 + 1000 gm
= 1245.85 gm
Volume of solution =
Ans. = 3 (the nearest integer)
New answer posted
6 months agoContributor-Level 10
All the solution have higher ion production with respect to 0.1 M C2H5OH i.e. 0.1 M Ba3 (PO4)2, 0.1 M Na2SO4, 0.1 M KCl and 0.1 M Li3PO4. Hence all have lowered freezing point than 0.1 M C2H5OH (which is non-ionisable in aqueous medium).
Ans. = 4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
