Solutions
Get insights from 202 questions on Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (ii)
Colligative properties are observed when a non-volatile solid or liquid are dissolved in a volatile liquid.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (iv)
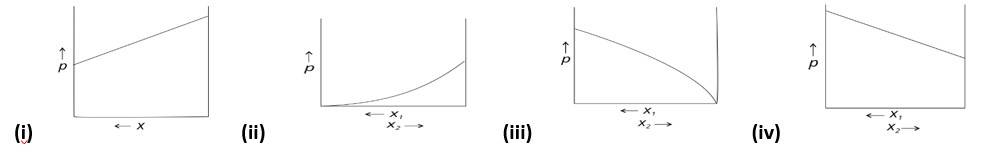
Explanation : Because the slopes in (i) and (iv) are straight lines, they represent the solution's ideal behaviour.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (iii)
For isotonic solutions osmotic pressure is same, solute or solvent may not be same.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (iii)
Explanation: At a specific composition Azeotropic mixtures of water, nitric acid, and ethanol have the same composition in the vapour and liquid phases.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (ii)
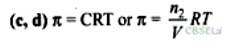
Isotonic solutions must have same osmotic pressure at a given temperature hence must have same volume and number of moles i.e., same molar concentration. Thus, the isotonic solutions have same elevation in boiling point, and depression in freezing point.
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (ii)
Explanation: Colligative property depends on
(i) The concentration of a nonelectrolyte solute in solution,
(ii) The number of particles of electrolyte solute in solution, & (iii) It does not depend on the nature of solute molecules/particles.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) and (iv)
Explanation: Intermolecular forces between benzene and toluene molecules in a combination of benzene and toluene molecules would be approximately as strong as those between two benzene molecules and two toluene molecules separately. As a result, the solution will form an ideal solution and follow Raoult's law. As a result, options (iii) and (iv) are false.
New question posted
8 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers