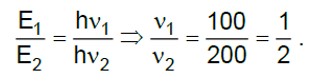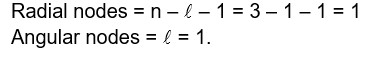Structure of Atom
Get insights from 126 questions on Structure of Atom, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atom
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
2 months agoContributor-Level 10
Power of bulb = 662 watt = 662 Js-1
Energy of one photon
Number of photons emitted
New answer posted
2 months agoContributor-Level 10
For a multi electron system, the energy of orbitals increase with increase in (n + l) value.
For same n + l value, the orbital with higher value of n has higher energy.
New answer posted
2 months agoContributor-Level 10
Yes, one can see the atoms, but not with the naked eye. One needs to use specialised microscopes to see the atoms. To see the atoms, one can use electron microscopes such as electron ptychography and TEM, or they can also use Scanning Tunneling Microscope (STM).
New answer posted
2 months agoContributor-Level 10
Helium (He) is the smallest atom. It has a smaller physical size as the protons in Helium create a stronger nuclear charge to pull its electrons closer to nucleus. The hydrogen is the lightest element.
New answer posted
2 months agoContributor-Level 10
In molecules, atoms are held together by the chemical bonds, that are strong attractive forces. The main two types of chemical bonds include ionic bond and covalent bond.
New answer posted
2 months agoContributor-Level 10
The following are the steps one must follow to draw the structure of an atom: First, find out the atomic number of the element on the periodic table, then draw a small central circle called the nucleus that includes the protons and neutrons. Next, there will be concentric circles around the nucleus for electron shells. Next, fill these shells with dots or crosses for electrons. One can fill around a maximum of 2 electrons in the first shell and a maximum of 8 in the second shell, and so on.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers