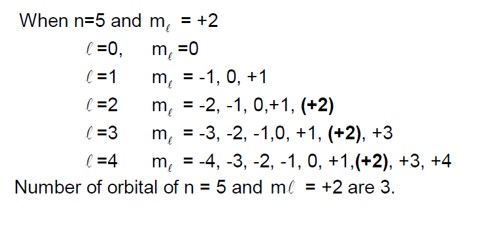Structure of Atom
Get insights from 126 questions on Structure of Atom, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atom
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
nm = 2l + 1
As there are (2l + 1) number of permissible values of magnetic quantum number.
Hence, l = (nm - 1)/2
New answer posted
3 months agoContributor-Level 10
An Atom has three fundamental particles-electron, proton and neutron. The mass of the electron is 9.10939 * 10? ³¹ kg. Neutrons and protons, both are collectively known as nucleons. All the isotopes of a given element show same chemical properties. Dalton's atomic theory, regarded atom as an ultimate particle of matter.
New answer posted
3 months agoContributor-Level 10
For irreversible expansion of an ideal gas under isothermal condition
ΔU = 0, ΔS (Total) ≠ 0
New answer posted
3 months agoContributor-Level 10
The number of Body centred unit cells in all 14 types of Bravais lattice unit cells is 3.
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 9
Bohr's theory accounts for the line spectrum of single electron species but Li? has two electrons. Bohr's theory fails to explain splitting of spectral lines in presence of magnetic field i.e. Zeeman effect.
New answer posted
3 months agoContributor-Level 9
Combustion of ethane: C? H? + 7/2 O? → 2CO? + 3H? O.
Moles of C? H? = 3g / 30 g/mol = 0.1 mol.
Moles of H? O produced = 0.3 mol.
Number of H? O molecules = 0.3 * 6.023 * 10²³ ≈ 18.06 * 10²².
So, x = 18.
New answer posted
3 months agoContributor-Level 9
Quantum Numbers and Orbitals:
o Total Node = n - 1 (l => Azimuthal Q.N)
o Radial Node = n - l - 1
o Angular Node: l
o If Angular node = 0, then l = 0, i.e., S orbital.
o If Radial nodes = 2, then n - l - 1 = 2.
o Substituting l = 0 gives n - 0 - 1 = 2, so n = 3.
o Therefore, the orbital is 3s.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers